The authors review the technologies that may help bioprocessing become a truly continuous operation and present case studies that could contribute to the integration of upstream and downstream platforms.
By Anurag Rathore, Nikhil Kateja, Harshit Agarwal, Abhishek Kumar Sharma
 Benefits of continuous processing have been widely demonstrated by a variety of manufacturing industries. With the growing demand for biosimilars and an ever-increasing pressure for reduction in manufacturing costs, the biotech industry seems to exhibit a flourishing interest in the development of continuous biomanufacturing systems. While a bulk of the research efforts thus far have been focused on making individual unit operations continuous, a few studies have explored the possibility of a continuous end-to-end biomanufacturing process. This article aims to review the efforts to make various unit operations continuous as well as the integration of these operations to make a truly continuous process.
Benefits of continuous processing have been widely demonstrated by a variety of manufacturing industries. With the growing demand for biosimilars and an ever-increasing pressure for reduction in manufacturing costs, the biotech industry seems to exhibit a flourishing interest in the development of continuous biomanufacturing systems. While a bulk of the research efforts thus far have been focused on making individual unit operations continuous, a few studies have explored the possibility of a continuous end-to-end biomanufacturing process. This article aims to review the efforts to make various unit operations continuous as well as the integration of these operations to make a truly continuous process.
Throughout the evolution of manufacturing, various industries have gradually shifted from batch to continuous processing as production technology matured. The list of industries that have adopted continuous processing models includes petroleum, steel, automobile, consumer goods, and food manufacturing (1). The key drivers for this evolution include rising demand, the desire for reduced processing costs, more stringent requirements for consistent quality, and most importantly, the requirement for higher productivity. The biotherapeutic industry is presently undergoing a similar transition (2).
The industry already has experience in terms of continuous upstream processing. Perfusion technology has been successfully used for the production of biotherapeutics for more than a decade. In the past few years, researchers have worked toward making various downstream unit operations continuous. While there is a plethora of emerging continuous bioprocessing technologies available in the market, there are almost no concrete examples of successful implementation and integration of a fully continuous process for biologic products. The true potential of continuous bioprocessing can only be realized after successful integration of all the unit operations (3). There have also been new efforts by various regulatory organizations to encourage continuous processing for the manufacturing of biopharmaceuticals. Both FDA and the European Medicines Agency (EMA) have changed their guidelines to reflect a change in the definition of a batch. Batch is now defined by the quantity of material rather than the mode of manufacturing (4). Industry is also taking steps toward a completely continuous system by collaborating with academia to build commercial continuous processing technologies. An example of this is the MIT-Novartis collaboration in continuous pharmaceutical production. In recent years, there have been publications reporting development of a fully integrated end-to-end commercial continuous process for the manufacturing of recombinant monoclonal antibodies (5).
This article reviews the historical efforts to make various unit operations continuous and provides an overview of the efforts that have been undertaken to piece together a truly continuous process comprised of numerous unit operations. The authors also present a few case studies of successful integration of these technologies.
Technology enablers for continuous manufacturing
While the biotech industry has extensive experience in continuous upstream processing, commonly called perfusion, there has also been extensive development of other separation technologies such as continuous chromatography, refolding, precipitation, cell lysis, filtration, and aqueous two-phase extraction (3). Table I presents a summary of recent efforts in the advancement of continuous upstream and downstream unit operations. Multiple tools are now available to design a continuous process, and researchers have demonstrated the tools’ successful use in a large variety of applications.
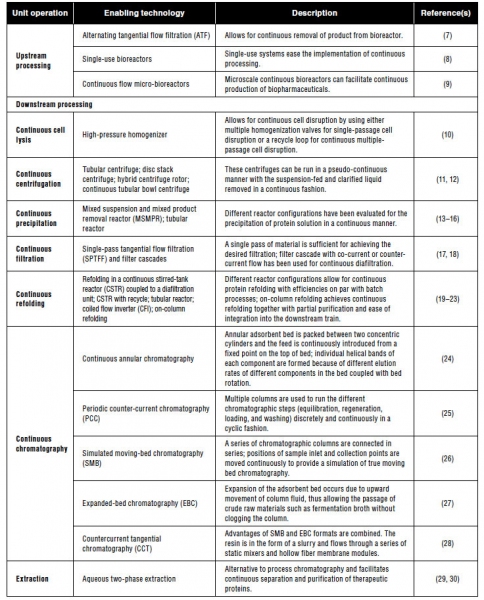
Table I: Summary of recent efforts to make various upstream and downstream unit operations continuous.
Prerequisites for designing a robust continuous process
Successful design of a robust continuous process requires a thorough understanding of the process with respect to successful identification of the critical process parameters and their interactions. Process development in the batch mode is the starting point; this is because if for the existing processes the batch protocol is already developed and validated, the motivation is to continue to use these existing batch protocols towards continuous process development. For instance, in the case of chromatography, the available batch protocol can be run using periodic counter-current chromatography (PCC) with modification made to the process parameters to match the requirements of continuous operation.
For the development of a new continuous process, the approach would be different than the aforementioned traditional technique. Continuous process development is focused on strategies to minimize intermediate sample conditioning to ensure a continuous flow of material between different unit operations. For instance, a process that has a fewer number of chromatographic steps and a continuous flow of sample between steps (no change in pH, conductivity adjustment, or both) is more desirable for operation in a continuous fashion. The increased use of multimodal resins and single-use technologies can also play an important role in continuous operation.
Individual efforts made by different researchers to make unit operations continuous (as depicted in Table I) collectively play a vital role when it comes to building an end-to-end continuous biomanufacturing process. A perfusion upstream process can only be used to deliver a completely continuous manufacturing process when it is coupled with other continuous downstream unit operations such as continuous centrifugation, filtration, and chromatography. Integration of the continuous upstream and downstream technologies, therefore, plays a vital role. Successful integration can result in a pseudo-steady state. This pseudo-steady state, however, refers only to the flows in and out of each unit operation. Gradients (e.g., concentration, pH, and conductivity) in these properties are expected in the effluent of several unit operations. These gradients can be largely decreased by selecting an appropriate volume of surge material to act as a buffer going into the next unit operation (6). In addition, the length of different unit operations also has to be matched to ensure continuous operation. Due to the complex nature of time balancing between different unit operations, there are several commercial simulation software programs that are available and can help to ensure effective time matching between chromatographic steps. Hence, incorporation of surge vessels and balancing the flow rates between different steps help to certify effective integration.
In summary, the key considerations for developing a continuous biomanufacturing process include a thorough process understanding, efficient integration of upstream and downstream unit operations, and the incorporation of a surge vessel and flow/time balancing.
Case Studies in continuous processing
Case study 1: Continuous refolding using a novel coiled flow inverter reactor (CFIR)
Researchers have proposed use of a novel CFIR for refolding of a granulocyte colony stimulating factor (GCSF) (22, 31). The configuration (Figure 1) has the ability to perform refolding at a much higher concentration and offers lower processing time compared with a corresponding batch process (22, 31). The dynamic mixer at the beginning of the reactor configuration provides the rapid mixing that is required during addition of reduced protein to the refolding buffer, and the CFIR provides constant mixing along with narrow residence time to keep the refolding mixture homogenized for the specified time. The CFIR was found to offer productivity 15 times higher than that produced through a batch process; this higher productivity was primarily due to the elimination of shutdown, cleaning, and filling steps in the continuous mode. It also offers an easy integration with other unit operations and flexibility of inline addition of additives. Moreover, the proposed unit can seamlessly be placed in a continuous bioprocessing train, taking input from an inclusion body solubilizing unit and providing the output to a suitable continuous purification step. Effectively, the configuration can significantly contribute to the development of an integrated continuous bioprocessing platform for bacterial recombinant proteins.
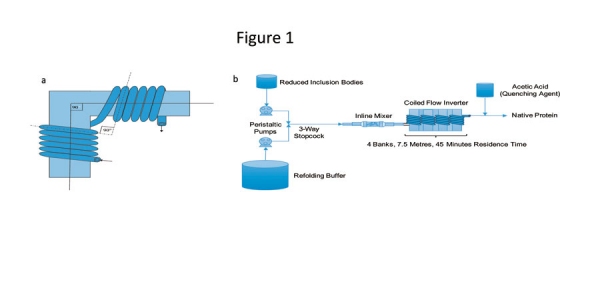 Figure 1: (a) Illustration of a bend for a coiled flow inverter (CFI). (b) Process flow diagram for the continuous refolding process. A bank is a collection of four branches, each with five turns of helix. Figures adapted from ref. 22.
Figure 1: (a) Illustration of a bend for a coiled flow inverter (CFI). (b) Process flow diagram for the continuous refolding process. A bank is a collection of four branches, each with five turns of helix. Figures adapted from ref. 22.
Case study 2: End-to-end fully integrated continuous process for production of recombinant monoclonal antibodies
As stated earlier, most efforts till date in the area of continuous biomanufacturing have targeted making the individual unit operations continuous. There are only a few cases where an entire process has been developed and all are for monoclonal antibody production. Researchers have recently demonstrated the first integration of high-density perfusion cell culture with a capture step through a four-column periodic counter-current chromatography (PCC) (1). The high-density Chinese hamster ovary (CHO) cells were operated at a quasi-steady stage for more than 60 days. The PCC system was directly integrated into this and ran continuously for 30 days. Product quality was found to be comparable with batch-column operation. The advantage of this assembly was realized in the form of elimination of several non-value-added unit operations, such as clarification and intermediate hold steps. In another publication, researchers extended the aforementioned work by further integrating the capture step with continuous intermediate and polishing steps (5). In that experiment, a continuous low pH hold was added to perform continuous viral inactivation. The whole assembly had the capacity to produce end-to-end continuous production from bioreactor to the polished product (Figure 2). The key distinguishing features of this facility were uninterrupted and fully automated purification, steady-state operation, and consistent product quality throughout operation time. In addition to eliminating several hold steps, the advantages of this platform were realized in the form of increased process throughput and decreased equipment footprint.
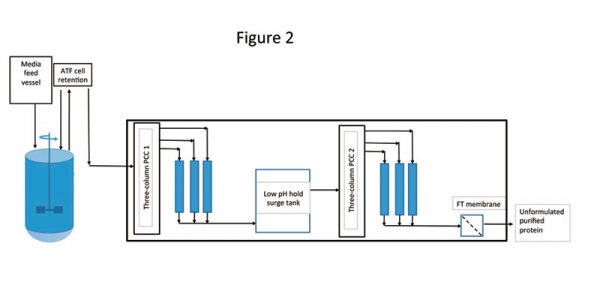
Figure 2: Architecture of end-to-end continuous bioprocessing. The architecture includes a perfusion bioreactor with alternating tangential filtration (ATF) as cell retention device for upstream processing. The downstream process included two three-column periodic counter-current chromatography (PCC) systems. Figure adapted from ref. 5.
Path forward
While the adaptation of continuous manufacturing strategies in biologics may have been slow, the field is evolving. The significant benefits that continuous processing offers—including higher profitability, ease of scalability, and lower manufacturing footprint—make this a topic of interest to every biomanufacturer. Researchers have proposed various new technologies for continuous operation, both in upstream and downstream processing. While improvisation at each step is important, future research needs to focus on integration of the multiple unit operations into a single process and optimization of the entire bioprocessing train as a whole. The success of continuous manufacturing technologies relies highly on the utilization of real time analytical tools coupled with superior control strategy. The implementation of process analytical technologies and quality-by-design approaches are bound to play a vital role in its success.
As is true for any new technology, adaptation for continuous biomanufacturing seems challenging and intriguing. The biotherapeutic industry is currently at the initial stage of new technology adaptation cycle and the benefits from continuous biomanufacturing will be evident in the coming years.
References
1. V. Warikoo et al., Biotechnol. Bioeng. 109 (12), pp. 3018–3029 (2012).
2. M.E. Kamarck, Nat. Biotechnol. 24 (5), pp. 503–505 (2006).
3. A.S. Rathore et al., Prep. Biochem. Biotechnol. 45 (8), pp. 836–849 (2015).
4. 21 CFR Title 21, Part 210.3 (Government Printing Office, Washington, DC).
5. R. Godawat et al., J. Biotechnol. 213, pp. 13–19 (2015).
6. M. Brower, Y. Hou, and D. Pollard, “Monoclonal Antibody Continuous Processing Enabled by Single Use,” chapter in Continuous Processing in Pharmacutical Manufacturing, pp. 255–296 (Wiley, Dec. 5 2014).
7. D. Voisard et al., Biotechnol. Bioeng. 82 (7), pp. 751–765 (2003).
8. W.G. Whitford and B.L. Pence, BMC Proceedings 7 (suppl 6), pp. P39 (2013).
9. L.D. Garza-García et al., Lab Chip 14 (7), pp. 1320–1329 (2014).
10. T. Sauer, C.W. Robinson, and B.R. Glick, Biotechnol. Bioeng. 33 (10), pp. 1330–1342 (1989).
11. C.F. Ivory et al., Biotechnol. Prog. 11 (1), pp. 21–32 (1995).
12. R. Lander, C. Daniels, and F. Meacle, Bioprocess Int. 11, pp. 32–40 (2005).
13. M. Raphael and S. Rohani, Chem. Eng. Sci. 51 (19), pp. 4379–4384 (1996).
14. M. Raphael and S. Rohani, Can. J. Chem. Eng. 77 (3), pp. 540–554 (1999).
15. P.D. Virkar et al., Biotechnol. Bioeng. 24 (4), pp. 871–887 (1982).
16. N. Hammerschmidt et al., Biotechnol. J. 10 (8), pp. 1196–205 (August 2015).
17. J.R. Alford et al., J. Pharm. Sci. 97 (8), pp. 3005–3021 (2008).
18. A. Jungbauer, Trends Biotechnol. 31 (8), pp. 479–492 (2013).
19. R. Schlegl et al., Chem. Eng. Sci. 60 (21), pp. 5770–5780 (2005).
20. S. Pan et al., Chem. Eng. Sci. 116, pp. 763–772 (2014).
21. A. Jungbauer, W. Kaar, and R. Schlegl, Curr. Opin. Biotechnol. 15 (5), pp. 487–494 (2004).
22. A.K. Sharma et al., Chem. Eng. Sci. 140, pp. 153–160 (2016).
23. S. Pan et al., J. Biotechnol. 185, pp. 39–50 (2014).
24. G.F. Bloomingburg et al., Ind. Eng. Chem. Res. 30 (5), pp. 1061–1067 (1991).
25. M. Kalyanpur, Mol. Biotechnol. 22 (1), pp. 87–98 (2002).
26. M. Juza, M. Mazzotti, and M. Morbidelli, Trends Biotechnol. 18 (3), pp. 108–118 (2000).
27. R.O. Owen and H.A. Chase, J. Chromatogr. A 757 (1), pp. 41–49 (1997).
28. O. Shinkazh et al., Biotechnol. Bioeng. 108 (3), pp. 582–591 (2011).
29. P.A.J. Rosa et al., Biotechnol. J. 8 (3), pp. 352–362 (2013).
30. P. Vázquez-Villegas, O. Aguilar, and M. Rito-Palomares, Sep. Purif. Technol. 141, pp. 263–268 (2015).
31. A.S. Rathore, K.D.P. Nigam, M. Pathak, H. Agarwal, and A.K. Sharma, (2015). Indian Patent No. 185/DEL/2015 (Provisional).
About the Authors
Anurag S. Rathore* (pictured) is a professor, Department of Chemical Engineering, Indian Institute of Technology, New Delhi, India. Nikhil Kateja, Harshit Agarwal, and Abhishek Kumar Sharma are all students at the Indian Institute of Technology Delhi.