Design of experiment is a powerful development tool for method characterization and method validation.
Design of experiments (DOE) is a well-proven characterization approach within product and process development and a key aspect of quality by design. Recently, more attention has been placed on applying DOE to analytical methods. DOE for analytical methods has three major applications: method development for new methods or those that need improvement, method validation, and quantitation of the influence of analytical methods on product and process acceptance and out-of-specification (OOS) rates. Method development seeks to understand where critical process parameters are in the analytical method and to minimize their influence on accuracy and precision. DOE for method validation seeks to validate the analytical method for a range of concentrations so that changes in formulation or concentration will not require additional validation as they are changes within a characterized design space. Once methods have been developed, qualified, and validated, the impact they have on OOS rates and process capability needs to be quantified and evaluated to determine their fitness for use.
A systematic approach for using DOE for analytical method development and validation is discussed in this paper and was written in line with the International Conference of Harmonization (ICH) Q2(R1), Q8(R2), and Q9 guidelines (1-3). A quantitative understanding of the factors that influence resolution, linearity, precision, and accuracy is integral to applying DOE to method development.
Textbook approaches to DOE generally suggest a sequential approach to DOE: screening studies, characterization studies, and optimization of the method or process. This approach applied to analytical methods is often not practical as 10-20 methods are often used for drug substance and drug-product evaluation and the amount of time and materials needed to follow the three steps (i.e., screen, characterize, and optimize) would consume unreasonable amounts of resources. The sequence generally recommended by the author for method development is understanding the purpose of the study, perform risk assessments to screen out factors that may or may not have an influence on the analytical method (screening variables by logic and an examination of their scientific potential for influence), and characterization studies to quantify and minimize their influence on precision, accuracy, and linearity.
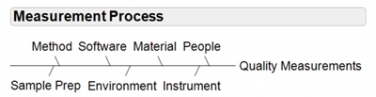
Assays and measurement systems must be viewed as a process (see Figure 1). The measurement process is made up of methods, standards, software, materials, chemistry, reagents, analysts, sample preparation methods, environmental conditions, and instrumentation/equipment. Quality risk management and statistical data analysis techniques should be used to examine the process of measurement and identify factors that may influence precision, accuracy, linearity, signal to noise, limits of detection and quantification, and/or any other assay attributes to achieve optimal assay results.