Development of Purification for Challenging Fc-Fusion Proteins
 Lab Photo/Shutterstock.comRecent advances in mammalian cell culture processes have significantly increased product titers, but have also resulted in substantial increases in cell density and cellular debris as well as process and product-related impurities.
Lab Photo/Shutterstock.comRecent advances in mammalian cell culture processes have significantly increased product titers, but have also resulted in substantial increases in cell density and cellular debris as well as process and product-related impurities.
Particularly for Fc-fusion proteins, these improvements have resulted in a new set of starting conditions for downstream processing, including elevated levels of high molecular weight species (HMWs; up to 20%) and low molecular weight species (LMWs; up to 20%). New advances in Fc-fusion downstream processing are crucially needed.
In this study, utilizing a combination of high-throughput condition screening in 96-well plates, optimization in small-scale column models, and a novel virus-spiking strategy, the authors have developed an alternative Fc-fusion protein polishing process that incorporates flow-through strategy in polishing steps using cation exchange or hydrophobic interaction chromatography.
As tested on three Fc-fusion proteins, this process demonstrates high process yield, improved clearance of HMWs and LMWs, and efficient reduction of host cell proteins (HCP) and DNA as well as robust viral clearance. Consistent performance of flow-through operations was demonstrated in multiple cGMP campaigns at the 2000-L scale.
Introduction
Improvements have been made in mammalian cell culture to achieve high productivity processes primarily to meet the increasing demand of biological therapies. In response, titers for monoclonal antibodies (mAbs) and Fc-fusion proteins have significantly increased (1-5).
Particularly for Fc-fusion proteins, these improvements have resulted in a new set of starting conditions for downstream processing, including elevated levels of high molecular weight species (HMWs; up to 20%) and low molecular weight species (LMWs; up to 20%).
Monoclonal antibody purification processes exist in different well-established platforms with extensive process performance histories for production of commercial mAbs (6-10), which typically consist of two or three chromatographic column steps for mAb purification that allow for the purification of similar molecules with minimal development and optimization.
In most two-column downstream processing platforms, the first column is Protein A (ProA), which binds the target mAb directly from the harvested cell culture fluid. A low pH buffer is routinely used to elute the mAb product, which is followed by a viral inactivation step.
Anion exchange chromatography (AEX) (6-8, 10-11), typically serves as a polishing chromatographic step operated in flow-through mode, binding process-related impurities such as HCP, DNA, leached ProA, endotoxins, viruses, and, in some cases, HMWs, while the mAb product passes through. However, traditional AEX is not effective at removing extremely high levels of HMWs (>20%), nor is it efficient for reduction of LMWs, which is frequently observed for Fc-fusion molecules.
In addition, Fc-fusions often pose significant challenges to other purification platforms using traditional cation exchange chromatography (CEX), hydrophobic interaction chromatography (HIC), or mixed-mode chromatography as a product polishing step.
Under normal bind/elute operating conditions, it is extremely difficult to remove both HMWs and LMWs while maintaining acceptable process yield. New advances in Fc-fusion downstream processing are crucially needed.
In this study, both CEX and HIC resins were evaluated in a flow-through mode as a polishing step in purification of Fc-fusion proteins that previously proved challenging to purify using conventional AEX, HIC, or mixed-mode chromatography.
Using a combination of high-throughput process development (HTPD) in a 96-well batch binding format and small-scale column optimization experiments in a flow-through mode, the authors have developed a Fc-fusion protein polishing step successfully implemented at large scale, which demonstrates acceptable step recovery and efficient clearance of impurities for three different Fc-fusion molecules.
For all molecules tested in the study, acceptable clearance of HCP, DNA, and leached ProA was demonstrated. Particularly for one of the molecules, the residual HMWs were reduced through CEX in a flow-through mode by 85% and LMWs by 91%. Additionally, more than 3.9 logs of viral reduction were observed during the viral clearance study using a model virus, Xenotropic murine leukemia virus (X-MuLV).
Lastly, the methods described here can be applied to the purification process development of other chromatography resins.
Materials and Methods
Purification techniques
Feed stock: Fc-fusion proteins with an isoelectric point (pI) ranging from four to seven were expressed in recombinant Chinese hamster ovary (CHO) cells and partially purified through ProA capture chromatography. The load for the Fc-fusion Protein A contained HMWs of up to 5% and LMWs at a range of 16 to 20%. The feed stream for Fc-fusion proteins B and C was a product of one additional chromatographic step. Fc-fusion protein B contained HMWs in the range of 10 to 25%. Fc-fusion protein C contained up to 8% HMWs.
High-throughput screening: All chemicals (MilliporeSigma) were of United States Pharmacopeia or multi-compendial grade. For each sub-step, 1 mL of solution was in contact with 100 µl of resin. Sixty percent resin slurry was dispensed into the 96-well format filter plate (Seahorse or Agilent) followed by equilibration at the target pH and conductivity.
Subsequently, protein was loaded into the plate at 40 mg/mL resin followed by sequential wash and strip. Buffers were formulated in an acetate buffer system of pH 4.0 to 5.5 with 0 to 250 mM sodium chloride (NaCl) concentration. The filter plate was shaken between stages at 1200 revolutions per minute (RPM).
Incubation time was 10 minutes for all phases with the exception of the sample application, which was incubated for one hour. The filtrate was captured in the collection plate stacked beneath the filter plate.
The final stage involved 1 M NaCl solution to strip the protein from the resin to allow calculation of the mass balance. The samples in the collection plate were then analyzed by an ultraviolet (UV)-vis spectrophotometer and electrophoresis on a chip (Perkin Elmer) in a 96-well format. All experiments were performed at room temperature.
Column chromatography and methods: CEX resins (Cytiva) and HIC resins (Tosoh Bioscience and Cytiva) were utilized during the experiments. All chromatography runs were performed using a Cytiva ÄKTA Explorer or Pure system installed with Unicorn software version 6. HIC and CEX columns were packed in a vantage 0.66-cm or above column (MilliporeSigma) with a bed-height of 10 to 20 cm and residence times of five to six minutes were maintained upon scale-up.
Equilibration with five-column volume was performed followed by varying the load-ratio conditions and chase with equilibration buffers. Fractions of the flow-through were collected during the loading and analyzed.
Data analysis
Statistical analysis was carried out using Minitab software. Percent reduction was defined through the use of Equation 1:
![]()
[Eq. 1]
Analytical techniques
Protein concentration was measured using a NanoDrop 2000 (Thermo Fisher Scientific). Microplate protein concentration analysis was measured using a Spectramax (Molecular Devices) plate reader.
Residual ProA levels were measured by enzyme-linked immunosorbent assay (ELISA) method for the quantitation of ProA ligand in solution (Repligen). Quantitation was achieved by comparing the signal of the sample to ProA ligand standards of known concentrations with a 0.1 ng/mL limit of quantification.
CHO HCP levels were measured by ELISA method for the quantitation of residual host cell proteins in solution (Cygnus Technologies). Quantitation was achieved by comparing the signal of the sample to host-cell protein standards of known concentrations with a limit of quantification of 2.5 ng/ml.
CHO DNA levels were measured by quantitative polymerase chain reaction (qPCR) method for the quantitation of host-cell DNA in solution (Thermo Fisher Scientific). Quantitation was achieved by comparing the signal of the sample to DNA standards of known concentrations with a limit of quantification of 25 pg/ml.
Measurement of aggregation levels was determined by size-exclusion chromatography (SEC) using ultra performance liquid chromatography (UHPLC) (Waters) with Empower software that used an Acquity UPLC BEH200 SEC column. The protein was monitored by UV280 nm.
LMW fragment quantification was performed using a method that involves the heating of IgG samples in the presence of sodium dodecyl sulfate (SDS) under non-reducing conditions. A capillary electrophoresis system (Beckman Coulter) applied high voltage to a capillary, and the electric charge caused separation by size using an UV detector to measure absorption.
High-throughput quantification of LMW fragment levels was achieved with a higher throughput analytical platform (Perkin Elmer). The analytes were -separated electrophorectically under non-reducing conditions, and the bands were detected using laser-induced fluorescence.
Results and Discussion
Resin and condition screening using 96-well filter plates
The major purification challenge for Fc-fusion Protein A was a high level of LMWs (up to 20%) observed in the eluate of the ProA capture step. In the absence of detailed characterization of the LMW impurities, different cation and mixed-mode chromatographic resins were screened during the initial phase of development.
It was quickly discovered that CEX in a flow-through mode was a potential method of separation between product and LMWs. In addition, the relatively low pI of the molecule makes it a good candidate for cation exchange chromatography.
The second round of HTPD was performed for one CEX resin under narrower operating ranges in a flow-through mode. The selected resin was a strong cation exchanger with a negatively charged sulfonate group in the ligand. Fc-fusion Protein A was screened under various pH and NaCl conditions. Greater than 90% overall process yield was observed in all the conditions evaluated. As expected, product flow-through was promoted in high salt and high pH conditions as indicated by the high yield in the flow-through pool (Figure 1A).
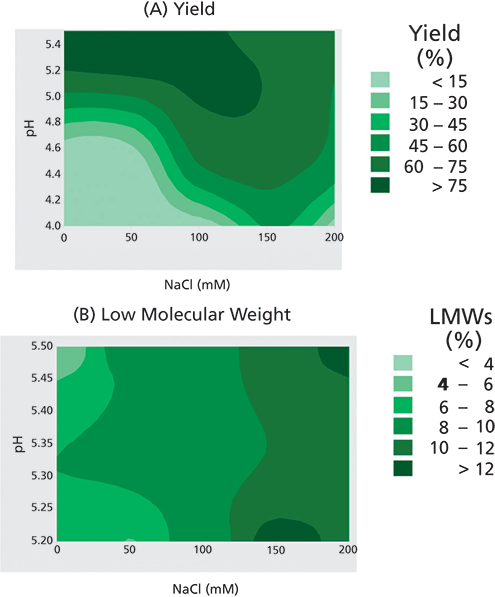 Figure 1. Cation exchange chromatography resin evaluated for the effect of operating conditions, pH, and sodium chloride concentration. Contour plots from screening were evaluated for two response parameters: (A) yield; (B) low molecular weight species with starting levels at 20%.
Figure 1. Cation exchange chromatography resin evaluated for the effect of operating conditions, pH, and sodium chloride concentration. Contour plots from screening were evaluated for two response parameters: (A) yield; (B) low molecular weight species with starting levels at 20%.
As shown in Figure 1B, there is a significant difference in the partitioning behavior between product and LMWs under the screening conditions. Appreciable reduction of LMWs was observed at 0-125 mM NaCl and pH 5.2-5.5.
Process capacity determination with column chromatography
The screening above with the desired purity was used as a starting condition for small-scale column experiments at a higher load ratio.
Eluate from the ProA capture step was adjusted to pH 5.0 and NaCl concentration adjusted to 80 mM with a concentrated NaCl stock solution. Following the equilibration, sample was loaded on a 0.66-cm inner diameter column to a bed-height of 13 cm. Loading was performed up to 300 mg/mL-resin. A residence time of six minutes was maintained for the load, and wash phases with product fractionation at intervals.
Figure 2 presents the product recovery versus the level of LMWs with a starting concentration of 18%. Breakthrough of the LMWs was observed at approximately 200 mg/mL-resin, indicating high process capacity for the LMWs.
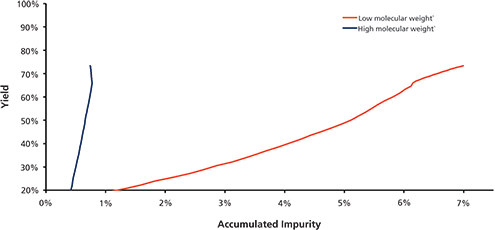 Figure 2. Process yield vs impurities (low molecular weight species [LMWs] and high molecular weight species [HMWs]). Under the cation exchange chromatography conditions tested, the efficient impurity removal was obtained up to 300 mg/mL-resin load ratio with starting HMWs level of 5% and LMWs of 18%.
Figure 2. Process yield vs impurities (low molecular weight species [LMWs] and high molecular weight species [HMWs]). Under the cation exchange chromatography conditions tested, the efficient impurity removal was obtained up to 300 mg/mL-resin load ratio with starting HMWs level of 5% and LMWs of 18%.
This high capacity can be attributed to the presence of non-charged groups interspaced within the spacer arm linked to the functional ligand on the resin. HMWs breakthrough was not observed throughout the run, suggesting a stronger binding of HMWs to resin than other product-related species.
In addition to purification of the monomeric product, process-related impurities were also efficiently reduced by flow-through CEX as the first polishing step. HCP was decreased by 60% and residual ProA level below 1 ppm. DNA was below the limit of quantification in the starting material for the study.
In summary, the primary parameters governing cation exchange chromatography, pH, and NaCl concentration were screened based on process performance such as product recovery, capacity, and selectivity. CEX steps are more typically used in a bind/elute mode, where impurities with more acidic species than the product may be removed in the load and wash, while more basic impurities are separated from the product during elution.
As a result of the flow-through CEX application with significantly higher load ratio (up to 400 mg/mL-resin in this case), buffer consumption is dramatically decreased while improving or maintaining the recovery of the target protein. This work evaluated the feasibility of implementing a flow-through CEX unit operation as a polishing step during purification of a challenging Fc-fusion protein.
HMW removal through HIC in a flow-through mode
In contrast to Fc-fusion A, the greatest purification challenge for Fc-fusion Proteins B and C was the HMW content in the feed stream. Similar to Fc-fusion Protein A, HMWs had not been characterized for Fc-fusion Proteins B and C prior to the study.
High molecular weight contents of the starting material were up to 25% for Fc-fusion Protein B and 8% for Fc-fusion Protein C, which were good candidates for HIC evaluation.
HIC is widely recognized as an effective method in reducing aggregated species. Resin selectivity is primarily based on the immobilized ligand and protein adsorption in an aqueous high-salt mobile phase and desorption in low-salt to no-salt buffer. The main parameters to consider when selecting HIC media and conditions are ligand hydrophobicity, salt type, and salt concentration. The feed stream was sourced from the first polishing chromatography step for Fc-fusion Proteins B and C.
Several resins, including butyl and phenyl, were screened (data not shown) using Fc-fusion protein B. The hydrophobic interaction resin with phenyl ligand was selected based on increased selectivity. Condition screening was performed for the phenyl with two salt types (NaCl and sodium citrate [NaCitrate]) and concentrations (NaCl, 750 to 1250 nM; NaCitrate, 100 to 200 nM). The study adhered to the flow-through mode of operation.
The feed was prepared by adjusting the counterion concentration to the specified concentration of the salt type with the addition of stock solution. Following the equilibration, sample was loaded on a 1.1-cm inner diameter column to a bed-height of 20 cm. Loading was performed to 100 mg/mL-resin. A residence time of five minutes was maintained for the load and wash phases with product factions were collected.
A decrease in yield with NaCl concentration was observed with Fc-fusion Protein B (Figure 3A). This phenomenon is interesting and has been reported as the “reverse salt effect” (12).
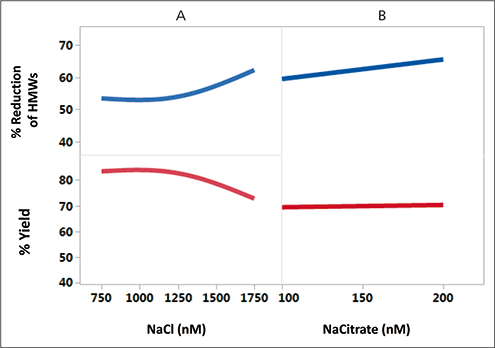 Figure 3. Yield and high molecular weight impurities reduction profiles at varying counterion concentration for hydrophobic interaction chromatography (HIC). Under the HIC conditions tested, robust yields and impurity removal were obtained under conditions containing sodium citrate (NaCitrate), and high reduction values were obtained at higher NaCitrate concentrations. HMWs is high molecular weight species.
Figure 3. Yield and high molecular weight impurities reduction profiles at varying counterion concentration for hydrophobic interaction chromatography (HIC). Under the HIC conditions tested, robust yields and impurity removal were obtained under conditions containing sodium citrate (NaCitrate), and high reduction values were obtained at higher NaCitrate concentrations. HMWs is high molecular weight species.
This reverse salt effect was mostly observed with ionizable matrix and was independent of the salt, which is indicative of non-specific binding. In this study, this effect is dependent upon the anion, indicating that the target protein also seems to be playing a role.
Figure 3B with NaCitrate shows a more robust operating space for process yield and reduction of the HMWs. Sodium citrate is chaotropic and tends to promote hydrophobic binding and enhance the solubility of proteins in solution. Because of this, NaCitrate with low counterion concentration was selected for better manufacturability.
A similar approach was used to determine the resin and conditions for Fc-fusion protein C with an increased column ratio of 180 mg/mL-resin. However, the effect of salt type was in agreement with Fc-fusion protein B (data not shown). A summary of a representative experimental run for three molecules and a cGMP 2000-L run for Fc-fusion protein B run is shown in Table I.
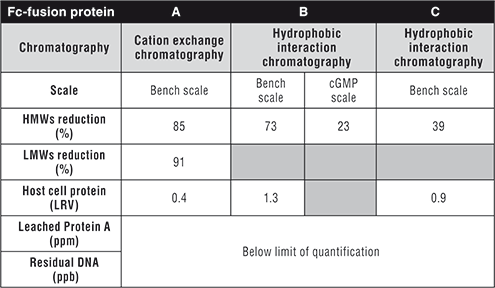 Table I. Summary of process performance and product quality for 3 Fc-fusion proteins using flow-through cation exchange chromatography or hydrophobic interaction chromatography. HMWs is high molecular weight species. LMWs is low molecular weight species.
Table I. Summary of process performance and product quality for 3 Fc-fusion proteins using flow-through cation exchange chromatography or hydrophobic interaction chromatography. HMWs is high molecular weight species. LMWs is low molecular weight species.
It should be mentioned that further optimization was performed for the first polishing step for Fc-fusion protein C before the HIC, which significantly reduced the HMW level in the starting material and increased robustness of the downstream operations. This may explain the lower HMW reduction fold observed for this molecule.
Discussion
Viral clearance is an important aspect when developing and optimizing downstream processes. However, there are very limited data available from flow-through CEX and HIC chromatography (13). The clearance capability was demonstrated with Fc-fusion Proteins A and B.
Initial viral clearance studies for Fc-fusion A using flow-through cation exchange with conditions previously identified revealed ≥ 3.9 log reduction value (LRV) for X-MuLV. Relatively limited clearance (1.5 LRV) was observed with mouse minute virus (MMV). Evaluation of HIC in a flow-through mode for its viral clearance capability using Fc-fusion Protein B was also -performed, which resulted in a clearance value of > 4.2 logs.
A flow-through mode unit operation is generally preferred over bind/elute in manufacturing processes. Advantages can include higher process capacity, smaller column size, shorter cycle time, and, in most instances, lower manufacturing cost. Smaller buffer volume is also typically required, which may confer an advantage if there is a limit on buffer tank capacity.
In addition, wider operating ranges can be defined relative to traditional bind/elute chromatography, which is likely to result in greater process robustness and manufacturing flexibility. As such, CEX or HIC chromatography in flow-through mode is likely to provide a valuable addition to Fc-fusion protein purification with challenging properties.
The demonstration of the viral clearance capability of these CEX and HIC chromatography methods in a flow-through mode, along with clearance of product and process-related impurities, paved the way in implementing these unit operations for clinical and commercial manufacturing of Fc-fusion proteins.
References
1. B.D. Follstad, R.E. McCoy and A.E. Morris/Amgen Inc., “Mammalian Cell Culture,” Patent application WO2013006479A2 [7], Jan. 10, 2013.
2. Y.K. Kang et al., Biotechnol. Bioeng. 110 (11), 2928-2937 (2013).
3. T.M. McNerney et al., “Flocculation Method,” Patent application WO2013090820A1[8], June 20, 2013.
4. X. Zhao et al., “Developing Recovery Clarification Processes for Mammalian Cell Culture with High Density and High Solid Content,” Presentation at the ACS National Meeting and Exposition (San Diego, CA, 2012).
5. I.C. Ikechukwu et al., “Method for Purifying Fc-fusion Protein,” Patent application WO2013009526A1 [9], Jan. 17, 2017.
6. B. Kelley, Biotechnol. Prog. 23(5), 995-1008 (2007).
7. A.A. Shukla et al., J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 848 (1), 28-39 (2007).
8. Y. Kang et al., BioPharm Int. 25 (5), 9 (2012).
9. H.F. Liu et al., mAbs 2 (5), 480-499 (2010).
10. Y.K. Kang et al., Pharm. Bioprocess. 3 (8), 477-487 (2015).
11. Y. Hou et al., Biotechnol. Prog. 31 (4), 974-982 (2015).
12. J. Chen et al., J. Chromatogr. A. 1177(2), 272-281 (2008).
13. G. Miesegaes, PDA J. Pharm. Sci and Tech 68(1) 30-37 (2014).
Author Biography
Ijeoma Cynthia Ikechukwu and Guodong Chen are senior scientists; Richard Ding is principal scientist; Evan Shave is associate director, Technology Transfer; Jay Jicao Kang is director of Analytical Development; and Yun (Kenneth) Kang*, yun.kang@patheon.com [10], is director of Process Development. All are at Patheon Biologics.
*To whom correspondence should be addressed.