QRM TOOLS
QRM tools are typically used in risk identification, risk analysis, evaluation, and control. There are two types of tools: risk assessment and control and statistical/analytical tools. Risk assessment and control tools aid in risk identification, risk control, and risk communication. Statistical/analytical tools aid in variation analysis, identification of risk areas, and determining the probability and frequency of potential risks. Analytical and quality tools further help to identify and prioritize risk. Knowing the right tool for the right job is fundamental to proper risk identification and control.
Risk-assessment tools include:
•FMEA
•Fault tree analysis (FTA)
•Hazard analysis and critical control points (HACCP)
•Hazard operability analysis (HAZOP)
•Preliminary hazard analysis (PHA)
Statistical/analytical tools include: •Pareto and risk-weighted Pareto analysis
•Process flow and risk priority numbers (RPN)
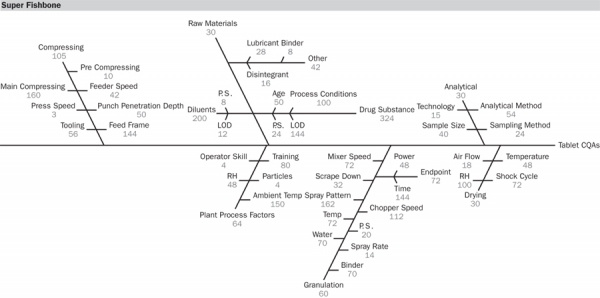
•Super Fishbone (Cause and effect diagram with RPN) (see Figure 8)
•Histograms and process capability
•Control charts
•Sniper plots
•ANOVA and hypothesis testing
•DOE (product and process)
•Variation studies, partition of variation (POV), and residual expected maximum likelihood (REML).
QRM IMPLEMENTATION
Every company that is involved in drug development needs to take QRM seriously. An implementation plan with tools, training, and standard operating procedures are needed to build a low-risk development and manufacturing platform. Management involvement is needed to trigger risk-assessment generation, form teams, communicate risk reduction or acceptance actions, and establish a formal review process to measure results and progress. Clarification of roles and responsibilities for QRM within a company also will need to be formalized.
Thomas A. Little, PhD, is president of Thomas A. Little Consulting in Highland, UT, drlittle@dr-tom.com.
REFERENCES
1. ICH, Q9 Quality Risk Management (ICH, 2006).
2. ICH, Q6 Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances (ICH, 2000).
3. ICH, Q8 Pharmaceutical Development (ICH, 2009).
4. ICH, Q10 Pharmaceutical Quality System (ICH, 2009).
5. ICH, Q11 Development and Manufacture of Drug Substances (ICH, May 2012).
6. FDA, PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance (Rockville, MD, 2004).