R eview gowning practices as a tool to evaluate cGMP compliance in a facility.
eview gowning practices as a tool to evaluate cGMP compliance in a facility.
The foundation of any successful quality-assurance system is strict adherence to good manufacturing practices (GMPs). Quality must be built into the design of a facility to ensure that drugs are manufactured under conditions and practices required by the GMP regulations. This concept is broadly embraced across the globe and promulgated by drug regulatory agencies, such as FDA in the United States, the European Medicines Agency in the European Union, and the World Health Organization, whose drug regulations are often used by developing countries and individual countries (e.g., China, India, and Brazil).
This principle is further enhanced by the practice of being current. FDA explains the idea of “current” as follows:
…the “c” in cGMP stands for “current,” requiring companies to use technologies and systems that are up-to-date in order to comply with the regulations. Systems and equipment that may have been “top-of-the-line” to prevent contamination, mix-ups, and errors 10 or 20 years ago may be less than adequate by today’s standards (1).
In a new facility, the expectation is that the design successfully embodies cGMPs. In existing legacy facilities and as buildings age, however, ensuring compliance with cGMPs is more complicated. A review of a facility’s gowning operations can bring significant insight into the current state of cGMP compliance.
Gowning embodies many of the major elements of cGMPs and as such is a window into a facility’s cGMPs. A highly compliant facility will have gowning operations that are clearly defined and readily understood (see Figure 1). Such operations are easy to diagram, easy to describe, and easy to apply consistently. Conversely, confusing, complicated, or highly individualized gowning operations are indicative of a facility with compliance issues. When gowning procedures are not simple or when there is a lack of consistency and gowning exceptions are allowed, a red flag should be raised and a detailed review of the facility undertaken.
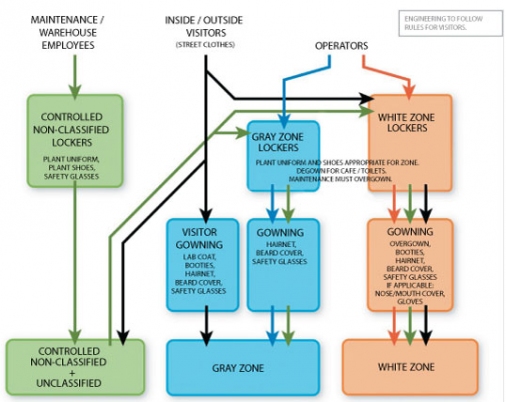
Because gowning is integral to a facility’s operation and reflects many of the fundamental aspects of cGMPs, an initial evaluation of a facility can be conducted by analyzing where and how gowning occurs. The following are three major gowning characteristics to look for.
Centralized vs. decentralized
Is gowning centralized or decentralized? Centralized gowning, occurring as part of the entry to a specific production area, although not a guarantee, is indicative of organized and orderly production zones. For centralization, functions of the same cleanliness grade must be grouped together and not dispersed into individualized or isolated islands of production. Centralized gowning is also indicative of organized material and personnel flows. If activities of one hygiene type are aggregated together, materials and personnel only need enter the zone once to have access to all the functions within. This can be contrasted with decentralized zones that require material and personnel to enter and exit production areas multiple times in the course of processing a single batch. Operational time is wasted on excessive material handling and multiple gowning/de-gowning sequences.
Appropriate gowning
Is the protective clothing appropriate for the cleanliness level of the area? In production areas designed to be classified as controlled environments (i.e., designed using ISO 14644-1 or Annex 1 of the EU GMPs [Manufacture of Sterile Medicinal Products]), the greatest source of contamination is the people working in the space. The clothing requirements must provide the amount of protection commensurate to the type of product processed, the operation being undertaken, and the cleanliness class or grade of the space.