Downstream Processing and Purity Analysis
For downstream laboratory-scale purification, performed using standard, non-disposable equipment, approximately 10 L virus harvest from the upstream processing was used. Approximately 44 L virus harvest from the 50 L (remaining 6 L corresponded to microcarrier volume) culture was used for further downstream processing with ReadyToProcess equipment in the scaled-up purification process.
Impurities originating from the host cells were removed during the downstream process. Cell debris was removed in the first microfiltration step using ULTA Prime GF 2 μm and 0.6 μm normal flow filters, followed by host cell DNA removal using a ReadyToProcess Capto™ Q chromatography column.
Host cell proteins were removed using a ReadyToProcess Capto Core 700 chromatography column.
Capto Core 700 chromatography medium (resin) was used in the second chromatography step of the purification process (6). This chromatography medium has an inert outer layer of cross-linked agarose preventing molecules with a molecular weight (Mr) larger than approximately Mr 700 000 to enter the core of the bead. The bead core contains octylamine ligands that bind a broad range of substances including proteins, peptides, and nucleotide fragments. The multimodal function of the chromatography medium, utilizing both size exclusion and affinity binding, reduces the number of purification steps needed by combining two procedures in one. The properties of octylamine ligand, allowing for binding of impurities over a broad pH range, high salt concentrations, and in various buffer compositions, enable the use of Capto Core 700 chromatography medium over a wide variety of conditions without impairment of its function. Hence, a sample from a previous purification step can be loaded directly on the Capto Core 700 medium column, without the need for pre-adjustment of buffer conditions. For our study, these benefits enabled decreased number of buffers needed. The possibility to serially connect Capto Q and Capto Core 700 media columns allows for enhanced system utilization and a reduced overall process time.
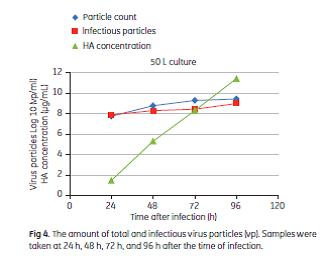
Before the sterile filtration step, the viral particles were concentrated and transferred into the final buffer by diafiltration using a ReadyToProcess hollow fiber cartridge (RTPUFP-500-C-9S). Finally, the solution was sterilized by filtration using ULTA Pure HC 0.6 μm/0.2 μm sterile filter. The virus content was determined by measuring the HA concentration by a Biacore method (7). The amount of infectious particles was analyzed by assaying 50% tissue culture infective dose (TCID50). The total amount of virus particles was determined by virus count (Virus Counter 2100, ViroCyt, Denver, CO, USA). Purity was determined by measuring host cell DNA (quantitative PCR), host cell proteins (Biacore method), and total protein (Bradford method).
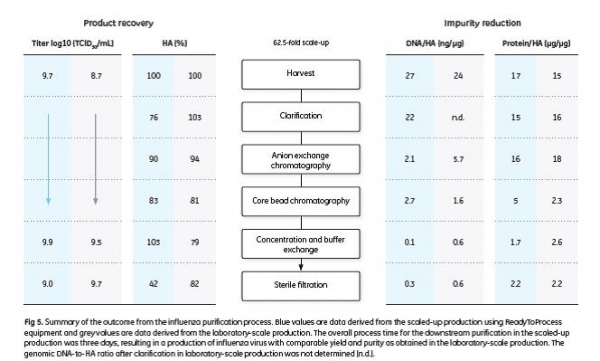
The results in terms of TCID50, HA yield, host cell genomic DNA-to-HA ratio, and total protein-to-HA ratio are displayed in Figure 5. The infectivity of the virus was retained throughout the process, as shown by the TCID50 titers. A more detailed description of the downstream purification process, virus count and purity analysis is given in the application note 29-0435-49 (5).
Comparison to regulatory specifications
As there is no approved cell-based LAIV on the market today, no regulatory requirements in terms of impurities are established. Hence, the output from the scaled-up production is compared to a commercially available specification for a nasal LAIV and a specification for egg-based, split-inactivated influenza vaccine from WHO (8). The study outcome is summarized in Table 1.
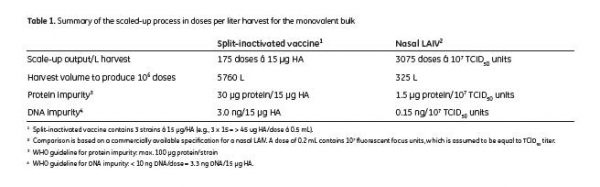
Assuming a nasal route of administration and doses of 107 infectious particles per 0.2 mL dose, the amount of host cell DNA in the scaled-up production was shown to be below the acceptance level (10 ng/dose) defined by WHO. The host cell protein amount per dose and strain in the scaled-up production was also shown to be below acceptance level of WHO. The outcome from the scaled-up production indicates that it is possible to obtain approximately 3000 doses/L harvest, corresponding to harvests of 325 L for 1 million doses (calculations based on specification for nasal LAIV) and 175 doses/L harvest corresponding to harvests of 5760 L for 1 million doses (calculations based on specification for split inactivated Vaccine.
Conclusions
This document describes a scaled-up production of influenza vaccine using ReadyToProcess single-use equipment. Many vaccine production processes are in a scale suitable for single-use equipment and could benefit from the increased flexibility and possibility for optimized facility utilization compared to using traditional stainless steel equipment. Single-use equipment enables quick changeover between products, minimizes risk for cross-contamination between batches, and reduces the need for cleaning and validation operations. This allows for an increase in the annual number of batches and multiproduct manufacturing, with an overall improved process economy as a result.
This case study shows that single-use equipment, including disposable cell culture bioreactors, pre-packed chromatography columns, and filters, can replace traditional equipment, including stainless steel bioreactors, user-packed chromatography columns, and ultracentrifuges, for the production of vaccines with high purity.
The case study described in this application note is not a fully optimized process. Further optimization of the process is necessary prior to use in vaccine manufacturing.
References
1. Global Action Plan for Influenza Vaccines (GAP). World Health Organization, Geneva.
[online] www.who.int/influenza_vaccines_plan/en/ (2011)
2. Partridgea, J. and Paule Kienyb, M. Global production capacity of seasonal
influenza vaccine in 2011. Vaccine 3:728-731(2013)
3. Pietrzykowski, M., Flanagan, W., Pizzi, V., Brown, A., Sinclair, A., and Monge M. An
Environmental Life Cycle Assessment Comparing Single-Use and Conventional
Process Technology. BioProcess Int 24:30-38 (2011)
4. Application Note: Scale-up of adherent Vero cells grown on Cytodex microcarriers
using ReadyToProcess equipment, Cytiva, 29-0435-48, Edition AA (2013)
5. Application Note: Downstream scale-up purification of influenza virus using
ReadyToProcess equipment, Cytiva, 29-0435-49, Edition AA (2013)
6. Application Note: Purification of influenza A/H1N1 using Capto Core 700 Cytiva, 29-0003-34, Edition AB (2012)
7. Nilsson, C.E., Abbas, S., Bennemo, M., Larsson, A., Hämäläinen, M.D., and Frostell-
Karlsson, A. A novel assay for influenza virus quantification using surface plasmon
resonance. Vaccine 28:759–766 (2010)
8. Recommendations for the production and control of influenza vaccine (inactivated).
World Health Organization Tech Rep Ser 927:103. (2005)
Ordering information
Related literature Code number
• Scale-up of adherent Vero cells grown on Cytodex microcarriers using ReadyToProcess equipment, Application note 29-0435-48
• Downstream scale-up purification of influenza virus using ReadyToProcess equipment, Application note 29-0435-49
• Microcarrier cell culture principles and methods, Handbook 18-1140-62