By Anurag S. Rathore, Destin A. LeBlanc
ABSTRACT
Quality by Design principles such as design space can also be applied to cleaning validation. As discussed in the recently published PDA Technical Report No. 49: Points to Consider for Biotechnology Cleaning Validation, well–designed laboratory-scale studies can be performed using design of experiments, and the data analyzed to understand the cleaning process. With the knowledge of large-scale equipment, one can create an approach that results in a successful cleaning validation.
 Cleaning validation plays an important role in reducing the possibility of product contamination from biopharmaceutical manufacturing equipment. It demonstrates that the cleaning process adequately and consistently removes product residue, process residue, and environmental contaminants from the cleaned equipment or system, so that this equipment or system can be safely used to manufacture subsequent products, which may be the same or a different product.PDA recently issued the Technical Report No. 49: Points to Consider for Biotechnology Cleaning Validation (1). This report, more than 70 pages long, was created by a team of European and North American professionals from biotechnology manufacturers, cleaning-chemical suppliers, regulatory agencies, and consulting companies. The report provides a single-source overview for biotechnology manufacturers and complements existing guidance and reference documents. It builds on and updates previous PDA publications, including the 1998 Technical Report No. 29: Points to Consider for Cleaning Validation (2) and the 1996 Technical Monograph, Cleaning and Cleaning Validation: A Biotechnology Perspective (3). The report uses a life-cycle approach to biotechnology cleaning validation that encompasses design and development, process qualification, and ongoing control of effectiveness. It also considers the risks of the process. In particular, this technical report addresses unique features of biotechnology cleaning validation, including the way in which limits are established for bulk biotechnology manufacture. The report also considers the effect of degradation of the active on cleaning-validation practices and the widespread use of nonspecific methods, such as total organic carbon (TOC) and total protein, for measuring residues of actives. Table I provides a list of the major topics covered in Technical Report No. 49.
Cleaning validation plays an important role in reducing the possibility of product contamination from biopharmaceutical manufacturing equipment. It demonstrates that the cleaning process adequately and consistently removes product residue, process residue, and environmental contaminants from the cleaned equipment or system, so that this equipment or system can be safely used to manufacture subsequent products, which may be the same or a different product.PDA recently issued the Technical Report No. 49: Points to Consider for Biotechnology Cleaning Validation (1). This report, more than 70 pages long, was created by a team of European and North American professionals from biotechnology manufacturers, cleaning-chemical suppliers, regulatory agencies, and consulting companies. The report provides a single-source overview for biotechnology manufacturers and complements existing guidance and reference documents. It builds on and updates previous PDA publications, including the 1998 Technical Report No. 29: Points to Consider for Cleaning Validation (2) and the 1996 Technical Monograph, Cleaning and Cleaning Validation: A Biotechnology Perspective (3). The report uses a life-cycle approach to biotechnology cleaning validation that encompasses design and development, process qualification, and ongoing control of effectiveness. It also considers the risks of the process. In particular, this technical report addresses unique features of biotechnology cleaning validation, including the way in which limits are established for bulk biotechnology manufacture. The report also considers the effect of degradation of the active on cleaning-validation practices and the widespread use of nonspecific methods, such as total organic carbon (TOC) and total protein, for measuring residues of actives. Table I provides a list of the major topics covered in Technical Report No. 49. 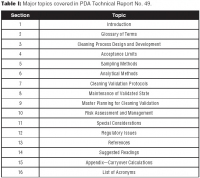
This article is the twenty-second in the "Elements of Biopharmaceutical Production" series and will discuss the various issues to consider when designing a cleaning-validation program.
CLEANING PROCESS DESIGN AND DEVELOPMENT
 Consistent with a life-cycle approach, a cleaning validation program should include the design of the cleaning process before its implementation in a manufacturing facility. A key to cleaning-process design is an understanding of the cleaning process itself, including critical quality attributes (CQAs) related to the outcome of the cleaning process, as well as critical process parameters (CPPs) of the cleaning process itself. The Technical Report discusses in detail the understanding of the various steps in a cleaning process. Table II illustrates considerations relating to CQAs and CPPs for cleaning processes. Table III illustrates considerations relating to cleaning-process design.
Consistent with a life-cycle approach, a cleaning validation program should include the design of the cleaning process before its implementation in a manufacturing facility. A key to cleaning-process design is an understanding of the cleaning process itself, including critical quality attributes (CQAs) related to the outcome of the cleaning process, as well as critical process parameters (CPPs) of the cleaning process itself. The Technical Report discusses in detail the understanding of the various steps in a cleaning process. Table II illustrates considerations relating to CQAs and CPPs for cleaning processes. Table III illustrates considerations relating to cleaning-process design.
 The four principal cleaning-input parameters for each step are sometimes referred to as time, action, concentration, and temperature (TACT). These four parameters can vary, but in a controlled cleaning process they are typically fixed. The exception is when principles of process analytical technology are used for process control. These parameters are also interrelated. For example, a cleaning process may be effective at a high temperature for a short time, and may be equally effective at a low temperature and a long time. The effect of these parameters on soil removal should be determined, with acceptable ranges established as part of the design and development effort. As the cleaning process is designed and developed, other issues, such as the appropriate residue-acceptance criteria and how to sample and analyze residue, should be considered.
The four principal cleaning-input parameters for each step are sometimes referred to as time, action, concentration, and temperature (TACT). These four parameters can vary, but in a controlled cleaning process they are typically fixed. The exception is when principles of process analytical technology are used for process control. These parameters are also interrelated. For example, a cleaning process may be effective at a high temperature for a short time, and may be equally effective at a low temperature and a long time. The effect of these parameters on soil removal should be determined, with acceptable ranges established as part of the design and development effort. As the cleaning process is designed and developed, other issues, such as the appropriate residue-acceptance criteria and how to sample and analyze residue, should be considered.
In addition, as part of the design and development effort, personnel should consider the various materials of construction used in biotech manufacturing. Laboratory evaluations of cleaning-solution compatibility (e.g., concentration, time, and temperature) and surfaces can be performed under simulated cleaning conditions. Differences between the cleaning of soils on those same surfaces also can be evaluated in the laboratory under simulated cleaning conditions.
These experiments enable employees to make determinations related to cleanability, such as comparing the equipment's materials of construction, comparing various soils for a given surface, and comparing various cleaning conditions (e.g., concentration of the cleaning agent, time, and temperature). Worst-case conditions (e.g., cleaning conditions less stringent than what is expected in the manufacturing equipment) may be employed in these laboratory evaluations. The outcome of these studies can be analyzed to create the design space for cleaning. The performance of the cleaning process in the laboratory is then verified by conducting experiments in the pilot-plant or scale-up equipment. Adjustments to cleaning conditions may be made during the scale-up process based on plant experience and laboratory development studies.
DEGRADATION EFFECTS
A key consideration in bioprocessing is that the active ingredient is usually degraded by cleaning processes that involve hot, aqueous, alkaline cleaning solutions. Although this degradation is a key mechanism of the cleaning process, and in many cases is required to remove denatured proteins from surfaces, it affects various elements of cleaning validation. For example, after a cleaning process, the active ingredient itself should not be present on cleaning surfaces; if residues are present, they may exist as degradation products of the active ingredient. Also, a specific analytical method for the active ingredient is not usually an appropriate analytical technique to determine whether the cleaning process is effective. These effects are discussed in more detail in Technical Report No. 49.
ANALYTICAL METHODS
Because of the degradation of the active ingredients in the cleaning processes for biotech manufacture, the most common practice is to use TOC as the analytical procedure to indicate the removal of the active. TOC also measures other sources of organic carbon, including media, cellular materials, detergents, and organic process materials. If total protein is used as a nonspecific analytical method for the active, that method may measure various protein species. Other methods, such as conductivity, may be used for the cleaning agent.
The appropriate analytical methods must be validated. Typically, validation involves principles from the International Conference on Harmonization's Q2 (R1) (4). Although degraded fragments of the active ingredient are measured in a cleaning-validation protocol, analytical method validation is typically performed with the bulk active itself because this typically reflects the worst case.
ACCEPTANCE LIMITS FOR ACTIVES
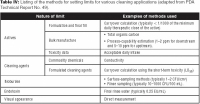 Personnel demonstrate the effectiveness and consistency of a cleaning procedure by showing that the cleaning process can reduce the amounts of potentially adverse residues to acceptable levels. For nonbiotech applications, limits for the active ingredient are typically established using a carryover calculation, which is based on the safety or toxicity of the active ingredient. For the manufacture of biotech products, however, that approach only works for fill–finish manufacture, where it is assumed that all measured organic carbon comes from the active ingredient (a worst-case assumption).
Personnel demonstrate the effectiveness and consistency of a cleaning procedure by showing that the cleaning process can reduce the amounts of potentially adverse residues to acceptable levels. For nonbiotech applications, limits for the active ingredient are typically established using a carryover calculation, which is based on the safety or toxicity of the active ingredient. For the manufacture of biotech products, however, that approach only works for fill–finish manufacture, where it is assumed that all measured organic carbon comes from the active ingredient (a worst-case assumption).
Table IV provides a list of the methods that can help set limits for the various cleaning applications (1). Limits for cleaning validation generally contain a measure related to the active protein or other major component of interest, a measure related to the cleaning agent, a measure related to bioburden levels, a measure related to endotoxin levels, and a requirement that the equipment be visually clean. In addition, if the active protein or other process components raise specific toxicity concerns (e.g., cytotoxicity, allergenicity, or reproductive hazards), the manufacturer's toxicology or pharmacology groups may determine whether limits should be modified or whether dedicated equipment is needed.
That approach does not work for limits for the active ingredient in bulk active manufacture. If the carryover limits are calculated using the entire equipment train's surface area, the limits are extremely low. If the active ingredient were undegraded after the cleaning process, it might be possible to measure the active ingredient using a specific analytical technique, such as enzyme-linked immunosorbent assay (ELISA). When the carryover limit for the active is converted to a TOC value, it typically is below a quantifiable TOC value for a swab or rinse value. That quantifiable value is close to 100 or 200 ppb carbon because of the background subtraction (i.e., the correction for the blank values).
A further complicating factor is that in the manufacture of the bulk active, residues left after earlier cleaning steps (e.g., until the first purification-process step) may be removed by subsequent purification processes, such as chromatographic purification. Therefore, consistent with ICH Q7, the cleaning of these earlier steps may not be critical for the carryover of residues to the final bulk active (5). Only a few literature references document the degradation of specific drug active proteins during the cleaning process. However, the literature contains ample evidence that proteins generally will degrade in hot, alkaline cleaning solutions. Although not well documented, this effect further mitigates the concern about carryover of residues in bulk active manufacture.
For these reasons, limits for the manufacture of bulk actives in biotech are generally established based on industry standard practice of about 5–10 ppm TOC for upstream processes and 1–2 ppm for downstream processes for any analytical sample, whether a swab sample or a rinse sample. The industry needs to provide more scientific rationales and data to support that practice, and such improvements in support documentation have started to occur.
SAMPLING METHODS
Another key part of a cleaning-validation program is appropriate sampling methods for the equipment surfaces and for the nature of the study, including the analytical methods used. The principles for sampling methods for biotech manufacture are not fundamentally different from those for sampling in nonbiotech cleaning validation. Sampling methods discussed in Technical Report No. 49 include "direct surface" sampling (e.g., using a fiberoptic probe), swabbing, rinse-water sampling, and placebo sampling. In practice, sampling for biotech manufacture may more likely involve rinse samples because much of the equipment is hard-piped and not readily accessible for swab sampling. Furthermore, some biotech companies like to use mock runs or blank runs (i.e., a type of placebo sampling) to provide an accurate picture of total carryover throughout the entire process of bulk active manufacture.
 Sampling recovery studies for biotech cleaning validation are not different in principle from sampling recovery studies in nonbiotech cleaning validation. However, residues used for spiking surfaces for recovery studies in biotech cleaning validation may include not only the bulk active, but also soils representative of early-stage harvesting steps. Furthermore, these recovery studies represent worst cases, in that residues actually sampled in cleaning-validation qualification protocols are actually degraded fragments of the active, which, being smaller in molecular weight and more polar, should be easier to remove in a sampling recovery study using water as the solvent. Table V provides some of the important considerations for choosing the sampling method for cleaning.
Sampling recovery studies for biotech cleaning validation are not different in principle from sampling recovery studies in nonbiotech cleaning validation. However, residues used for spiking surfaces for recovery studies in biotech cleaning validation may include not only the bulk active, but also soils representative of early-stage harvesting steps. Furthermore, these recovery studies represent worst cases, in that residues actually sampled in cleaning-validation qualification protocols are actually degraded fragments of the active, which, being smaller in molecular weight and more polar, should be easier to remove in a sampling recovery study using water as the solvent. Table V provides some of the important considerations for choosing the sampling method for cleaning.
MAINTENANCE OF VALIDATED STATE
A key part of the validation life cycle for any system is maintaining the validated state on an ongoing basis. Any change in the validated state of a cleaning process might detract from the quality, safety, and purity of manufactured products. Tools for validation maintenance covered in Technical Report No. 49 include change control, risk-based periodic monitoring, and data trending review. Training and retraining for manual cleaning processes are also significant because they are the primary mechanisms for obtaining consistency in manual cleaning processes.
For biotech and nonbiotech cleaning validation, actual values for residues (e.g., in rinse-water samples) are significantly below the acceptance criterion limit. The reason is that most manufacturers design their cleaning processes with a reasonable margin of safety so that any samples taken during qualification protocols or during routine maintenance will pass the acceptance criteria with a good margin of safety (e.g., a robust cleaning process is designed). Therefore, the fact that actual residue values are significantly below acceptance limits should not by itself be a reason for making qualification protocol limits more stringent. This situation is often addressed by establishing action or alert levels for residues for routine monitoring samples. Routine monitoring results above such action or alert levels provide an indication of a possible change in the cleaning process, thus requiring an investigation into the cause.
ANALYTICAL METHODS
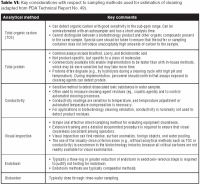 Appropriate analytical methods are essential to the success of cleaning validation. They need to be able to adequately detect the residues of concern. Table VI summarizes the key considerations that apply to some of the most common analytical methods in cleaning validation. Specific analytical methods (e.g., high-performance liquid chromatography and ELISA) measure a certain residue in the presence of expected interferences. Interferences may include degradation products and related substances, excipients, cleaning agents, and cleaning agent byproducts. In contrast, nonspecific analytical methods (such as TOC, Bradford, conductivity, and visual inspection) measure a general property, such as conductivity or TOC, which could result from various analytes or sources.
Appropriate analytical methods are essential to the success of cleaning validation. They need to be able to adequately detect the residues of concern. Table VI summarizes the key considerations that apply to some of the most common analytical methods in cleaning validation. Specific analytical methods (e.g., high-performance liquid chromatography and ELISA) measure a certain residue in the presence of expected interferences. Interferences may include degradation products and related substances, excipients, cleaning agents, and cleaning agent byproducts. In contrast, nonspecific analytical methods (such as TOC, Bradford, conductivity, and visual inspection) measure a general property, such as conductivity or TOC, which could result from various analytes or sources.
CLEANING-VALIDATION PROTOCOLS
Cleaning-validation protocols, like process-validation protocols, should include purpose, scope, responsibilities, applicable products and equipment, cleaning standard operating procedures, acceptance criteria, and a requirement for a final report. Key technical elements include residue limits, sampling procedures and analytical methods.
MASTER PLANNING FOR CLEANING VALIDATION
The master plan for cleaning validation should provide a description of responsibilities and activities for the planning and execution of cleaning validation. It should describe the overall plan, rationale, and methodology for cleaning validation. The plan should provide a high-level description of the cleaning-validation philosophy and strategy that will support the validation activities performed at the site. Detailed procedures for how cleaning validation is executed should be included in individual protocols. The plan will define the efforts required to ensure that the cleaning program complies with current good manufacturing practices. The validation activities are documented according to the requirements of the plan to provide sufficient scientific rationale to assess the suitability of the cleaning program to consistently clean equipment to the required specifications.
RISK MANAGEMENT AND ASSESSMENT
Quality risk management (QRM) involves elements of risk assessment, risk control, and periodic review to ensure continuous and effective control (6). It is important to achieve a shared understanding of the application of risk management among diverse stakeholders. Successful implementation of QRM requires support of the whole team, including operations, technical services, engineering, quality control, quality assurance, and regulatory personnel. This support is essential to identifying and addressing conditions that affect CPPs and CQAs for the cleaning or manufacturing process.
CONCLUSION
This article has reviewed the key issues in biotech cleaning validation. The authors encourage all biotech manufacturers to consult PDA Technical Report No. 49 for a detailed perspective on current practices and issues in biotech cleaning validation.
Anurag S. Rathore* is a biotechnology chemistry, manufacturing, and controls consultant and a faculty member at the Indian Institute of Delhi, India, +91 9650770650, asrathore@biotechcmz.com
. Rathore is also a member of BioPharm International's editorial advisory board. Destin A. LeBlanc is the principal consultant at Cleaning Validation Technologies.
*To whom all correspondence should be addressed.
Explore about this topic: Read, "Developing Cleaning in place protocols."
REFERENCES
1. PDA, Technical Report No. 49: Points to Consider for Biotechnology Cleaning Validation (PDA, Bethesda, MD, July 2010).
2. PDA, Technical Report No. 29: Points to Consider for Cleaning Validation (PDA, Bethesda, MD, Nov. 1998).
3. PDA, Technical Monograph, Cleaning and Cleaning Validation: A Biotechnology Perspective (PDA, Bethesda, MD, Dec. 1995).
4. ICH, Q2 (R1) Validation of Analytical Procedures: Text and Methodology, Step 4 version (2005).
5. ICH, Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients, Step 4 version (2000).
6. ICH, Q9 Quality Risk Management, Step 4 version (2005).
--Note: This article was first published in BioPharm International's March 2011 issue, and the original posting can be found here.