Mar 01, 2015
By Anurag S. Rathore, Mili Pathak, Guijun Ma, Daniel G. Bracewell
BioPharm International
Volume 3, Issue 28
In the past two decades, Protein A affinity chromatography has remained the de facto capture step for purification of monoclonal antibody products (mAbs). Protein A is of Staphylococcal origin and is expressed as a type I membrane protein in the bacterium Staphylococcus aureus. Its high specificity for the Fc region of antibodies is the primary reason for its dominance as the affinity ligand of choice in numerous immunological and purification applications (1). Specificity of Protein A ligand towards mAb results in extremely effective clearance of host cell proteins (HCP), viruses, and DNA (2–5). The high selectivity is accompanied by reasonably good physiochemical stability. The Protein A ligand has been attached to a large variety of different base matrices such as cross-linked agarose, surface modified porous glass, coated polystyrene, hydrogel filled into a ceramic shell, and other materials based on organic polymers. Thus, Protein A is considered to be the most crucial step in the purification process and a major contributor towards achieving the stringent purity levels expected of biotech therapeutics with HCPs reduced to <100 ppm and DNA to <10 pg/dose (6).
A typical Protein A chromatography process consists of five steps: pre-equilibration and equilibration of the column; capture of the mAbs from the harvest; wash step with the selective buffer to remove non-binding impurities; elution at low pH and conductivity; and finally, cleaning and sanitization of the column for next use. The widespread applicability and similarity of operating conditions have made Protein A chromatography the core purification operation in platform purification processes for antibodies (7, 8). Large-scale implementation of Protein A chromatography, however, offers several challenges (2, 9). In a typical platform process for production of mAb therapeutics, approximately 80% of the total process costs are for the steps following fermentation, with up to 60% of the downstream costs coming from chromatography. The most significant contributor to the total cost is the Protein A resin. The cost of Protein A resin is nearly 50% higher than traditional chromatographic media with nonproteinaceous ligands (2, 10). Resin cost for a large column (>1-m diameter) can exceed $1 million. What makes the cost of Protein A resin even more challenging is the fact that most antibody therapeutics require frequent and large doses. Hence, the resulting demand for the drug can be several hundred kilos of product per year or more (10).
Another important consideration for bioprocessing applications is throughput (11, 12). Because of the high expense of the resin, manufacturers need to recycle the resin over an extended period, often stretching their use for more than 100–200 cycles. Further, instead of using a large Protein A column, a smaller column is typically used and multiple cycles are performed on the smaller column during processing of a single batch of mAb. This cycling, however, increases the total purification time and thereby decreases process throughput.
In view of the previous discussion, there is a perennial need for Protein A resin that can offer high-binding capacities at high-flow rates so as to enable higher process throughput. It is also important that the resin is chemically and physically stable to last the desired number of re-uses. Over its long lifetime, the resin is subjected to extreme fluctuations in pH and conductivity during processing, cleaning, and sanitization. The cleaning and sanitization steps use harsh conditions to strip away any bound adventitious agents and to return the resin as close to its original state as possible. It has been shown that the extended use of a resin results in decrease in its binding capacity, a decrease in product recovery, and a loss of Protein A ligands (9). Recent innovations in Protein A-affinity chromatography have targeted creation of new resins capable of delivering high dynamic binding capacity and high throughput with better mass-transfer properties (e.g., EMD Millipore’s ProSep-vA Ultra and ProSep A Ultra High Cap; and Cytiva’s MabSelect Xtra). These resins have been designed to keep pace with increasing bioreactor volumes and cell-culture expression levels and thereby prevent Protein A capture from becoming a bottleneck in the purification process.
In this thirty-second article in the “Elements of Biopharmaceutical Production” series, the authors present the outcome of a survey that was recently performed jointly by researchers at Indian Institute of Technology Delhi and University College London. The survey targeted a total of 15 major manufacturers of mAb products, including five Indian manufacturers. The objective of the survey was to understand the current industrial experience with regard to Protein A chromatography.
Resin type
Commercially available Protein A resins vary with respect to the source of the Protein A ligand (‘‘natural’’ wild-type vs. ‘‘recombinant’’), base matrix composition, bead size, and pore size. These attributes can result in differences in binding capacity, resin compressibility, chemical resistance, permeability, available surface area, and mass-transfer properties, all of which significantly affect the performance of the Protein A column (13). Some of the major Protein A resins that are commercially available include MabSelect (Cytiva), MabSelect Xtra (Cytiva), MabSelect SuRe (Cytiva), MabSelectSuRe LX (Cytiva), ProSep Ultra Plus (EMD Millipore), Poros MabCapture A (Life Technologies), AbSolute High Cap (Novasep), CaptivA (Repligen), and Toyopearl AF-rProtein A-650F (Tosoh Biosciences). Figure 1 illustrates the percentage of users for each type of resin as determined by the survey. MAbSelect media are all based on a highly cross-linked agarose gel matrix to withstand higher flow rates (14). MAbselect Xtra uses the same backbone chemistry as MAbSelect but has a wider pore size to improve mass transport and thereby the dynamic binding capacity (15). The decrease in surface area due to the larger pores is compensated by an increase in ligand density. The controlled pore glass-based Protein A resin from EMD Millipore (ProSep) has better flow characteristics and diffusional properties than agarose-based Protein A resins (13). However, it suffers from the disadvantage of reduced surface area for binding because of larger pore diameters. MabSelect SuRe consists of an engineered Protein A ligand with homotetramer-Z domain, where a number of asparagine residues have been replaced to eliminate interactions with the variable region, thereby reducing the binding heterogeneity between antibodies and allowing the resin to be able to withstand stronger alkaline conditions. This alkali resistance is an important advantage as it allows for repeated use of 0.1–0.5 M NaOH for cleaning and sanitization (16, 17, 18, 19). While the base matrices and ligand densities for the MabSelect and MabSelect SuRe are identical, the latter provides greater stability under alkaline conditions used in clean-in-place (CIP) protocols.
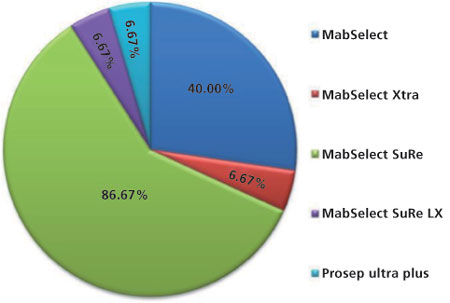 Figure 1: Pie chart illustrating type of Protein A media used for monoclonal antibodies/Fc-fusion protein manufacturing.
Figure 1: Pie chart illustrating type of Protein A media used for monoclonal antibodies/Fc-fusion protein manufacturing.
Number of cycles
As discussed previously, biotech manufacturers typically perform multiple cycles per batch to allow them to use a relatively smaller Protein A column than would otherwise be required. These cycles significantly reduce the capital cost of the column and the resin. Figure 2 illustrates the results from the survey on the number of cycles currently achieved compared with the number of cycles the respondent desires from a Protein A resin. Of the companies that participated in the survey, the maximum number of cycles that are currently used is between 150–200, with most using the resin for 50–100 cycles. While approximately 35% manufacturers are happy with the resin delivering 50–100 cycles, the same number also expect more than 200 cycles from the resin.
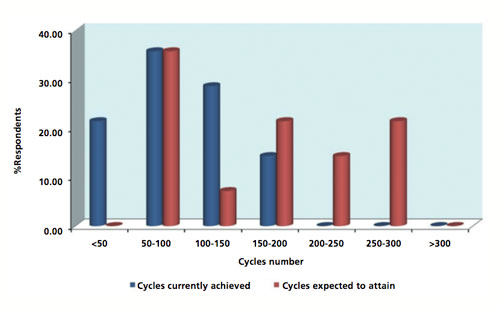 Figure 2: Bar chart illustrating the number of cycles currently achieved compared with the number of cycles the respondent would expect from a Protein A resin.
Figure 2: Bar chart illustrating the number of cycles currently achieved compared with the number of cycles the respondent would expect from a Protein A resin.
Monitoring of resin performance
It is well known that the efficacy of the chromatographic resin changes with use and as a result of it being stored for long periods of time (19). It has been observed by several researchers that the performance attributes of chromatographic stationary phases such as step yield, host-cell-impurity clearance capability and DNA-clearance capability are likely to degrade over time (9). An additional concern is that the ability of chromatography columns to clear adventitious viruses may also be reduced with extended use (20). In view of this, there is a need to continuously monitor performance of a chromatography column during manufacturing so as to be able to anticipate any performance gaps and take appropriate action to avoid column failure. Typical parameters that are used for column monitoring include yield, height equivalent to theoretical plate (HETP), impurities in eluate, product quality, and chromatographic profile (19–21). More recently, advanced approaches based on chemometrics have been used to monitor column performance (22, 23).
In the survey, participants were asked for the parameters that they monitor routinely as well as the level of importance of each parameter. Figure 3 presents the outcome. While all five of the parameters are used in the industry, yield is considered as the most important, followed by impurity levels, product quality, chromatographic profile, and HETP. Other than these five parameters used to measure host cell proteins, host-cell DNA, aggregates, charge variants, leached Protein A, and elution volume are also monitored during Protein A chromatographic runs.
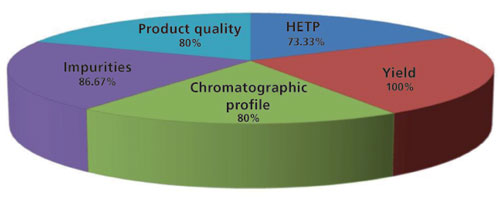 Figure 3: Parameters monitored routinely to track performance of Protein A resin during manufacturing.
Figure 3: Parameters monitored routinely to track performance of Protein A resin during manufacturing.
Large pores enable rapid mass transfer but also reduce the surface area available for the protein to bind, resulting in lower equilibrium capacities. High ligand densities result in high capacities but reduced mass transfer since the immobilized Protein A is a large molecule and reduces the pore diameter when immobilized in excess (24). To determine the performance loss of a Protein A resin, dynamic binding capacity (DBC) is the best and most widely used measurement (1, 24, 25). DBC describes the amount of sample that will bind to a resin packed in a column under defined conditions. It is highly dependent on operating conditions, sample preparation, and sample origin. Approximately 80% of industrial respondents have observed a decrease in DBC and flattening of the slope of the breakthrough curve as the cycle number increases, particularly with MabSelect SuRe. For other resins, the respondents have reported a decrease in DBC with a sharpening of slope of the breakthrough curve as the cycle number increases. Along with a decrease in resin binding capacity, respondents also observed discoloration and eluate peak broadening.
Users employ a variety of analytical tools to monitor Protein A resin deterioration over time. These include lysophosphatidic acid (LPA) analysis and HETP measurement, as well as monitoring of bioburden, endotoxin, host cell proteins, and media components in the pool over resin lifetime.
Factors affecting resin underperformance
Underperformance of a Protein A resin over re-use can be a result of ligand leaching or degradation, occludence of the pores with a reduction in available surface area, or as the result of deterioration of the packed bed from resin damage or fouling (26). Proteases in harvested cell culture fluid are known to be responsible for proteolytic cleavage of Protein A ligand into fragments ranging in molecular weight from six to 40 kDa (27). Protein A ligand on the resin can undergo nonspecific, noncovalent binding with the IgG and thereby result in detachment from the resin and co-elution with the antibody during elution. Also, dissolution of the base matrix can occur during pH cycling (ProSep A groups of products are known to be susceptible to this under certain conditions), resulting in leaching of the Protein A ligand. Finally, physical or chemical breakage of the glycosidic linkages in the agarose base matrix can occur in the MabSelect group of products (28, 29).
The results of the survey on resin re-use are displayed in Figure 4. Two of the most significant indicators of resin underperformance are the level of HCP in the pool (73%) and level of specific proteases (66%). Other significant attributes include the level of Protein A leachate in the pool, concentration of DNA in the pool, growth media composition, and the amount of lipids in the pool (Figure 4A).
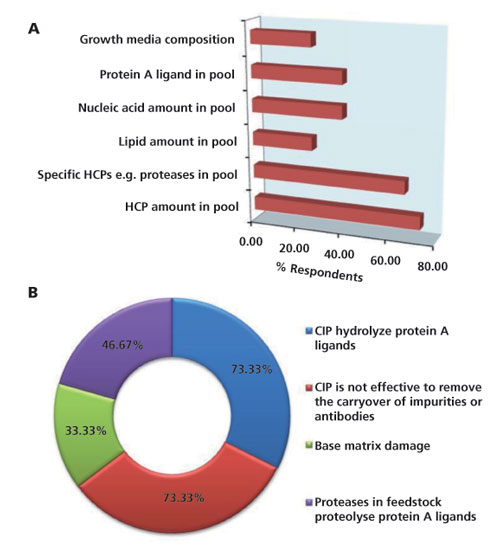 Figure 4: A) Attributes of Protein A column that may be affected by resin re-use. B) Factors that influence lifetime of Protein A resin.
Figure 4: A) Attributes of Protein A column that may be affected by resin re-use. B) Factors that influence lifetime of Protein A resin.
With respect to the factors that impact the lifetime of Protein A resin, the most significant factor is the selection of CIP solution. The impact of the CIP buffer is both with respect to the effectiveness of the CIP in removal of the impurities that bind to the resin as well as the hydrolysis of the ligand that can occur during CIP. Levels of proteases in the feed material has also been highlighted as a significant issue as it is believed that these proteases can result in loss of ligand via proteolysis. Finally, physical damage of the resin over re-use was also highlighted as a limiting factor by a few of the participants. Other factors that limit resin lifetime and were mentioned by the participants include changes in mass transfer characteristics of the resin, increase in back-pressure during operation, and an inability to remove lipids over an extended number of cycles.
Other factors affecting selection of protein A resin
The choice of Protein A resin may differ based on the stage of the product (clinical vs. commercial) as well as the number of batches that need to be manufactured in a year. For products where a large number of batches are being produced in a year, a resin with a long lifetime would be economical even if the cost of the resin itself were high. For products where only a few batches are being manufactured in a year, a resin with low cost but perhaps smaller lifetime may be more optimal (30). Overall, the survey suggested that there may be opportunity for manufacturers to come up with more economical Protein A resins with extended lifetime.
Summary
The survey provides a detailed insight into the Protein A resins used in the industry, the lifetime of a typical resin, the parameters tested to determine a typical lifetime, and the factors limiting a resin’s lifetime. Maximizing the lifespan of preparative Protein A columns is a central topic during process development for monoclonal antibodies and Fc fusion proteins. It is clear that the industry does not have a deep, fundamental understanding of the factors that influence performance loss and therefore, the ability to monitor and control degradation of the Protein A ligand and the base matrix is restricted. The current practice to establish media lifetime involves performing laborious cycling studies in a lab followed by continued monitoring in a plant. A better understanding of the mechanisms could result in smarter monitoring and control strategies and reduce the vast experimentation uncertainties.
Acknowledgements
The authors gratefully acknowledge support from the DST-EPSRC project DST/RC-UK/12-AM/2012.
References
1. R. Hahn, R. Schlegel, and A. Jungbauer, J. Chromatogr. B 790 (1), pp. 35-51 (2003).
2. D. K. Follman, and R. L. Fahrner, J. Chromatogr. A 1024 (1), pp. 79-85 (2004).
3. R. Valdés, N. Ibarra, and I. Ruibal et al., J. Biotechnol. 96 (3), pp. 251-258 (2002).
4. K. Brorson, J. Brown, and E. Hamilton et al., J. Chromatogr. A 989 (1), pp. 155-163 (2003).
5. K. Seop, Y.W. Choi, and S.R. Lee et al., J. Microbiol. Biotechnol. 11 619 (2001).
6. M. D. Butler, B. Kluck, and T. Bentley, J. of Chromatogr. A 1216 (41), pp. 6938-6945 (2009).
7. A. A. Shukla, B. Hubbard, and T. Tressel et al., J. of Chromatogr. B 848 (1), pp. 28-39 (2007).
8. Y. Yigzaw, R. Piper, and M. Tran et al., Biotechnol. Prog. 22 (1), pp. 288-296 (2006).
9. P. Gagnon, “Protein A affinity chromatography,” in Purification Tools for Monoclonal Antibodies, (Validated Biosystems, Tucson, AZ, 1996), pp. 155-158.
10. S. Ghose, B. Hubbard, and S. M. Cramer, Biotechnol. Bioeng. 96 (4), pp. 768-779 (2007).
11. R. L. Fahrner, H. V. Iyer, and G. S. Blank, Bioprocess Eng. 21 (4), pp. 287-292 (1999).
12. P. Jandera, D. Komers, and G. Guiochon, J. of Chromatogr. A 760 (1), pp. 25-39 (1997).
13. J. T. McCue, G. Kemp, and D. Low et al., J. of Chromatogr. A 989 (1), pp. 139-153 (2003).
14. Cytiva, “MabSelect,” 2006.
15. Cytiva, “MabSelect Xtra,” 2011.
16. Cytiva, “MabSelect SuRe: Alkali-stabilized protein A derived medium for capture of monoclonal antibodies,” November 2004.
17. S. Gülich, M. Uhlén, and S. Hober, J. of Biotechnol. 76 (2), pp. 233-243 (2000).
18. Cytiva, “MabSelect SuRe—studies on ligand toxicity, leakage, removal of leached ligand, and sanitization,” Application Note (2004).
19. A. S. Rathore and G. Sofer, “Lifespan studies for chromatography and filtration media” in Process Validation, A. S. Rathore and G. Sofer, Eds. (Marcel Dekker, 2005), pp. 169-203.
20. A. S. Rathore, “Qualification of a Chromatographic Column: Why and How to Do It,” BioPharm International, March (2003), pp. 30-40; Pharmaceutical Technology Europe, March (2003), pp. 45-56.
21. J. Moscariello, E. Lightfoot, and A. S. Rathore, BioPharm International 18 (8) (2005).
22. A. S. Rathore, S. Mittal, S. Lute, and K. Brorson, Biotechnol. Prog. 28 (5), pp. 1308-1314 (2012).
23. Y. Hou, C. Jiang, and A. A. Shukla et al., Biotechnol. Bioeng. 108 (1), pp. 59-68 (2011).
24. R. Hahn, P. Bauerhansl, and K. Shimahara et al., J. Chromatogr. A 1093 (1), pp. 98-110 (2005).
25. R. Hahn, K. Shimahara, and F. Steindl et al., J. Chromatogr. A 1102 (1), pp. 224-231 (2006).
26. C. Jiang, J. Liu, and M. Rubacha et al., J. Chromatogr. A 1216 (31), pp. 5849-5855 (2009).
27. J. N. Carter-Franklin, C. Victa, and P. McDonald et al., J. Chromatogr. A 1163 (1), pp. 105-111 (2007).
28. L. Schwartz, “Immunomodulatory properties of Protein A,” in Bacterial Immunoglobulin—Binding Proteins, M. Boyle, Eds. (Academic Press, San Diego, CA, vol. 2, 1990), pp. 309-318.
29. S. Vunnum, G. Vedantham, and Hubbard, “Protein A-Based Affinity Chromatography,” in Process Scale Purification of Antibodies, U. Gottschalk, Eds. (John Wiley & Sons, 2009), pp. 79-102.
30. J. Pollock, G. Bolton, and J. Coffman et al., J. Chromatogr. A 1284, pp. 17-27 (2013).
About the Authors
Anurag S. Rathore* (pictured) is a professor, Department of Chemical Engineering, Indian Institute of Technology, New Delhi, India.
Mili Pathak is a graduate student, Department of Chemical Engineering, Indian Institute of Technology, Delhi, India.
Dan Bracewell is associate professor, Department of Biochemical Engineering, University College London, London, UK.
Guijun Ma is a postdoctoral fellow, Department of Biochemical Engineering, University College London, London, UK.
*To whom all correspondence should be addressed.
ALL FIGURES ARE COURTESY OF THE AUTHORS
Article Details
BioPharm International
Vol. 28, No. 3
Pages: 28-33
Citation: When referring to this article, please cite it as A. Rathore, et al., “Re-use of Protein A Resin: Fouling and Economics,” BioPharm International 28 (3) 2015.