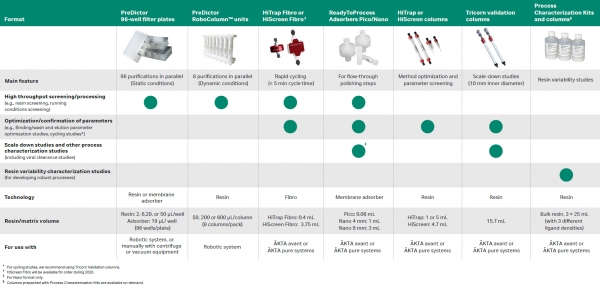
Development of robust purification processes requires significant time and resources. The quality-by-design initiative by the FDA puts additional demands on process development work because a higher degree of process understanding is needed.
Therefore, the choice of tools is critical to keep development time short, while increasing the amount of information available and keeping sample consumption low.