Products approved last year included four monoclonal antibodies (mAbs), four hormones, three blood factors, three subunit vaccines, as well as two each of colony stimulating factors (CSFs), fusion products, and enzymes. Five products are indicated against cancer, again rendering it the single most common target indication. A further three aim to treat various forms of haemophilia, while the remainder target a wide range of additional medical conditions.
In terms of expression systems used, a total of eight of the products approved are expressed in mammalian cell lines (seven in CHO cells, one in Sp20), six are produced in yeast-based systems, four in E. coli and two in insect-based systems. These approvals reflect trends in expression systems used over the past several years, with no novel expression systems making a debut in 2013.
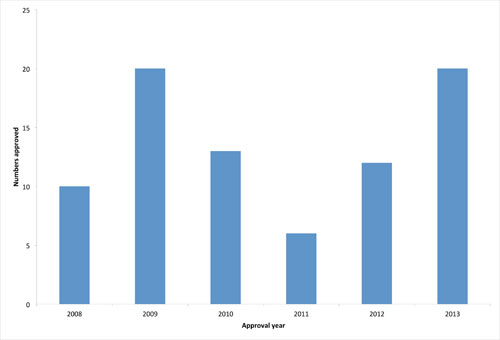
Twenty approvals, by any metric, is a healthy headline number. Digging a little deeper, however, reveals that just over two-thirds of these (a still credible 14 products) were genuinely new first time approvals. Six of the products, although newly approved in one region last year, had gained approval prior to that in the other region (Table I). Moreover, a significant difference in the absolute approval numbers was witnessed in the regions. A total of 16 approvals were recorded in Europe, while only six biopharmaceuticals came on the market in the US in that same period. This trend can partly be explained by Europe playing catch up in the case of some products; five of Europe’s 16 new approvals were already on the US market prior to 2013.
Overall, 2013 can be considered quite a successful year in terms of new product approvals. Several of those newly approved products underpin particularly noteworthy technical, medical, or regulatory milestones, and are thus the focus of the remainder of this article.