Application of multiple techniques at different conditions presents a more complete picture of a dynamic situation.
By Rebecca Strawn

Sergey/stock.adobe.com
Almost all drug products, whether they are small molecules or biologic in nature, will require some level of comparability studies to be performed. Whether they are called ‘sameness’ for generic drugs, ‘comparability’ for biologics, or ‘analytical similarity’ for biosimilars, these tests are crucial for any regulatory submission, which is vital to the entire product development process.
Standard testing routines comprise a selection of experimental techniques chosen because of their ability to monitor various quality attributes of the molecule. These are typically among those listed in the International Council for Harmonization (ICH) Q6B guidelines (1), although it is also important to note that complex generic APIs generally call for more creative analytical solutions. The quality attributes that are assessed include higher order structure for macromolecules, which involves the characterization and comparison of secondary, tertiary, and quaternary structures.
There are several standardized techniques used for the elucidation of secondary, tertiary, and quaternary characteristics of macromolecules. For secondary structural characterization, for example, common industry-standard techniques are far-ultraviolet circular dichroism (CD) and Fourier transform infrared spectroscopy (FTIR). While these two techniques are orthogonal, they are also complementary in that they are biased counter to one another: CD is biased toward reporting on α-helical content; and FTIR is biased toward reporting on β-sheet content. Similarly, for reporting on quaternary structure, analytical ultracentrifugation (AUC) and size-exclusion chromatography with multi-angle static light scattering (SEC-MALS) are commonly used orthogonal techniques, with respective over-reporting and under-reporting of oligomeric species. Instrumental techniques are often selected based on familiarity, commercialization, and efficiency. While these are all good criterion for routine method selection, the techniques themselves are not necessarily aptly suited to specific molecules or systems.
Each molecule and/or formulation system can present unique obstacles. For example, the sequence of the molecule may preclude certain techniques, or make other techniques difficult because of the size or molecular weight of the product. Similarly, the formulation buffer may include excipients that cause some techniques to fail, or the formulation concentration of the API may be too low to detect using certain techniques. These occasions may lead to the inability to truly build any orthogonality into the high order structure analytical comparability.
When these issues are encountered, it is key to empirically define the critical parameters of the available techniques in order to use each of them to the fullest advantage.
One criterion that is crucial for demonstrating suitability of a given technique is its ability to exhibit being “fit for purpose”; specifically, the method should be able to demonstrate specificity, or the ability to distinguish between non-degraded and degraded samples. However, despite this recommendation appearing in both EU and US guidelines for comparability studies, it is still common to see methods being used under conditions that are unlikely to lead to differences being observed between the products undergoing testing. For example, it is common for the samples to be tested using ill-suited techniques, often at a singular testing condition such as at room temperature (20 °C to 25 °C), which will only give a narrow view of the system. These experimental approaches do not fully take into account the complex nature of macromolecules.
Proteins are dynamic molecules that do not exist in a single conformation, but as a complex distribution of an ensemble of different conformations. This dynamic conformational landscape will commonly have a complex system of local energy minima under different conditions. As a result, while several different production batches of a protein may have a similar conformation when assessed at the same temperature, if the temperature changes, the conformational populations may structurally diverge from each other as they explore their independent dynamic landscapes. Therefore, if macromolecules are characterized and compared at different temperatures, the comparability assessment will be more reliable. Comparability should at the very least be evaluated at both the room temperature industry standard, and also at 37 °C, which is more biologically relevant.
Case study: preliminary peptide analysis
In this example, a medium-sized peptide was investigated. This system presented challenges due to the small size and limited structure of the peptide. Two orthogonal, industry-standard structural characterization techniques were explored and assessed for suitability.
The first technique employed was CD. Samples were prepared to approximately 0.2 mg/mL in an appropriate buffer, and CD measurements were made using a Jasco J-815 CD spectropolarimeter; the spectra were analyzed for secondary structural composition using the CDsstr (2) program. While the small size of the peptide likely precludes structural relevance to the fitting values, they can serve as a comparative metric between samples. The spectra suggest that, while the samples show general similarity above about 25 °C, the alpha-helix and beta-sheet content changes below this temperature (Figure 1). This may suggest that CD can report on sample differences as a function of temperature, thereby demonstrating specificity.
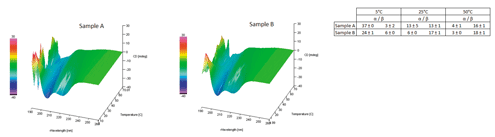
Figure 1. Circular dichroism (CD) spectra (left) of peptide samples and the abridged cdsstr2004 structural fitting results at select temperatures. The data suggest that while the samples show general similarity above ~25°C, below this temperature, the structural composition in regards to alpha-helix and beta sheet content changes significantly. (Figures courtesy of the author)
Experiments were also conducted on a Bruker Tensor 37 FTIR instrument with a photovoltaic MCT detector, using an AquaSpec cell; structural fitting was done via the vendor-specific software Opus. Buffer-subtracted spectra were generated by acquiring a background measurement of the formulation buffer immediately prior to spectrum acquisition for each sample. At all temperatures investigated, the structural components of the FTIR samples were similar (Figure 2).
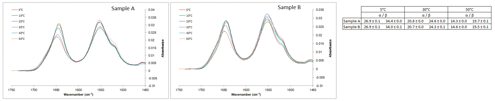
Figure 2. Transmission Fourier transform infrared spectroscopy (FTIR) spectra (left) of peptide samples and the OPUS structural fitting results at select temperatures. At all temperatures investigated, the structural components of the samples were similar. This is in contrast to the CD data and reveals both the possible limitations of certain experimental techniques and the possible advantages to orthogonal techniques. (Figure courtesy of the author)
These results are in contrast to those observed with CD, which may highlight the potential limitation of this experimental technique and may indicate that FTIR is less sensitive at discerning structural differences.
While a full method qualification of specific quality attributes requires much more statistical rigor, this preliminary case study demonstrates that exploring a broader landscape may add valuable comparative power. It also illustrates that exploring orthogonal techniques, and indeed, letting them ‘compete’ with one another, can enhance experimental design, especially for products challenging the resilience of a given methodology and/or technique.
The importance of multiple conditions
It is clear from these studies of the same peptide—at different temperatures, using different techniques—that there was divergence in higher order structure. These results highlight the inherent dynamic nature of peptides and proteins and illustrate the need for increasing the experimental scope of comparability studies. In fact, the regulatory agencies are continuously looking for more rigorous analytical strategies to have greater confidence in the obtained results, and to ensure accurate and reliable conclusions can be drawn from comparability/sameness/biosimilarity studies.
Testing biologic molecules under multiple conditions greatly benefits study design. It gives multiple testing points within a single experimental technique, thus expanding its comparative power. This is especially beneficial in those instances when a system cannot be tested using multiple orthogonal techniques because of experimental limitations. Using appropriate conditions would also lead to the generation of representative forced degraded forms, which are necessary to establish the specificity of any analytical methodology.
It also gives an insight into the dynamic landscape of the material and allows a more complete characterization to be made at various temperatures, or a range of other conditions. This can aid in understanding the different degradation pathways that might be in play, another factor that is a key element of a comparability study.
Overall, the application of multiple techniques at different conditions will give a far better understanding of the molecule and its biological activity and will mitigate the risks of obtaining restricted information when taking a snapshot at a single set of conditions. This approach may give a more complete picture of a dynamic situation.
References
1. ICH, Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products (ICH, March 10, 1999), www.ich.org
2. W. Curtis Johnston, Proteins 35 (3) 307–312 (May 1999).