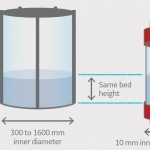
Effective viral clearance studies remain one of the challenges facing manufacturers of biologics. These studies are an essential part of process validation and are critical to ensure drug safety. Viral clearance studies are usually performed using a scale-down model mimicking the large-scale process step. For chromatography steps, using a scale-down model limits the amount of sample and resin used, lowering the cost for the study. As viral clearance studies are often cost and time consuming, it is essential to minimize risk of failure.