In cell-based influenza vaccine production, the European Pharmacopoeia demands a host cell residual DNA concentration of less than 10 ng per dose. To reliably measure residual DNA in both process samples and final vaccine by quantitative PCR (qPCR), DNA preparation prior to analysis is a necessity. Samples from the vaccine purification process contain different buffers, salts, and host cell compounds, and varies 3–4 logs in DNA concentration from harvest to the final product, which all put strain on the DNA preparation method.
For accurate determination of DNA concentration, recovery is of high importance. There are many commercially available DNA preparation kits that use different techniques to bind DNA, from spin columns with a DNA-binding membrane or resin to magnetic beads. However, these kits are developed mainly for purification of DNA fragments from gel electrophoresis or genomic DNA from tissues such as blood or cultured cells, and do not have recovery as a priority. Few kits are intended for residual DNA determination in samples with high concentration of a protein or virus product.
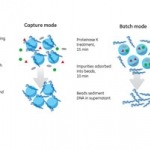
In this study, prototype chromatography resins for DNA preparation, in capture and batch modes, were evaluated for recovery, hands-on time, and throughput. In batch mode, recoveries of > 80% were achieved, but the technique exhibited matrix effects on real process samples. In capture mode, recoveries of 40%–60% were achieved after elution. However, recovery could be improved by concentration determination of DNA bound to the resin.