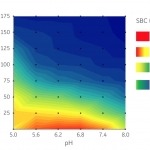
When establishing purification processes for a biosimilar molecule it is important to develop steps that are scalable, selective and cost effective.
This example describes a standardized workflow using a highthroughput process development (HTPD) approach: chromatography resin and running conditions were selected based on experience and adapted to meet requirements of biosimilars.