Key Process Considerations for Therapeutic Oligonucleotides
September 9, 2021 - Cytiva
Single-Use Technologies Prove Effective for Viral Vector Process
August 30, 2021 - BioPharm International
The benefits of single-use technologies for upstream viral-vector processes clearly outweigh their disadvantages.
Cynthia A.
…
How Do I Fix Asymmetrical Chromatography Peaks?
August 30, 2021 - Cytiva
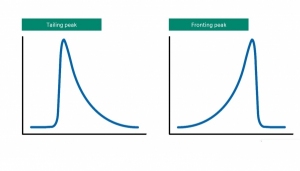 All good chromatography books say that
All good chromatography books say that
How to Optimize and Scale Up AAV Production
August 30, 2021 - Cytiva
Sanitization of AxiChromâ„¢ Columns Packed with Chromatography Re
August 30, 2021 - Cytiva
 …
…
Achieving the Required Buffer Quality with Inline Conditioning
August 30, 2021 - Cytiva
 …
…
Cell-free Expression Systems Pose Cell Culture Alternative
August 30, 2021 - BioPharm International
Cell-free expression is promising in preclinical applications, but still presents challenges to scale up for commercial production.
Novavax Reports on Two Vaccine Efficacy Studies
July 29, 2021 - BioPharm International
 Novavax’s C
Novavax’s C
Troubleshooting Protein Purity Issues – Top Tips
July 29, 2021 - Cytiva
 …
…
Cytiva and Pall Corporation Investing 1.5 Billion USD Over Two Ye
July 29, 2021 - Cytiva