Ensuring Viral Safety of Viral Vaccines and Vectors
September 27, 2018 - BioPharm International
Ensuring Viral Safety of Viral Vaccines and Vectors
…
FDA Seeks to Enhance Manufacturing of Cell and Gene Therapies
September 27, 2018 - BioPharm International
FDA Seeks to Enhance Manufacturing of Cell and Gene Therapies
…
Combining Purification Techniques in a Multistep Approach
September 13, 2018 - Cytiva
Videos! SPR Applications in Protein Characterization
September 13, 2018 - Cytiva

Integrated Semi-Continuous Process for mAb Production
September 5, 2018 - Cytiva
 A complete process for the production of a biopharmaceutic
A complete process for the production of a biopharmaceutic
Sensitive and Reproducible SPR-Based Concentration and Ligand-Bin
September 5, 2018 - Cytiva
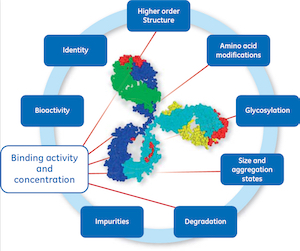 …
…
A Look into the Future of Biopharmaceutical Quality
September 5, 2018 - BioPharm International
A Look into the Future of Biopharmaceutical Quality
…
Optimizing Late-Stage and Commercial Cell-Culture Processes
August 16, 2018 - BioPharm International
Optimizing Late-Stage and Commercial Cell-Culture Processes
…
The Challenge of Disruptive Technologies in Bioprocessing
August 16, 2018 - BioPharm International
The Challenge of Disruptive Technologies in BioprocessingIncreasing demand for
…
Process Economic Simulation for Scalable Production of Adenovirus
August 16, 2018 - Cytiva
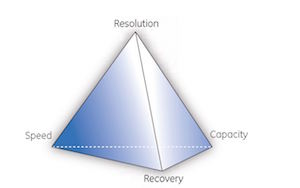
 This
This