GMP Challenges for Advanced Therapy Medicinal Products
December 3, 2015 - Pharmaceutical Technology
Finalizing GMP requirements and quality standards for the development, manufacture, and clinical testing of ATMPs in the EU is proving to be a complex t
…
Antibody Purification Handbook
December 3, 2015 - Cytiva
 The diversity of the antibody-antigen interaction
The diversity of the antibody-antigen interaction
CMOs Continue to Improve Overall Biomanufacturing Performance
November 19, 2015 - BioPharm Intl.
Better process development is creating industry benchmarks for bioprocessing.
By Eric Langer
…
Selecting the Right Viral Clearance Technology
November 19, 2015 - BioPharm Intl.
By Cynthia Challener, PhD

Automated harvesting and 2-step purification of unclarified mamma
November 5, 2015 - ScienceDirect
 Therapeutic monoclonal antibodies represent one of the fastest
Therapeutic monoclonal antibodies represent one of the fastest
A Q&A With Günter Jagschies: Recovery of Biological Products Con
November 5, 2015 - Cytiva
Process Development Forum speaks with Günter Jagschies, Cytiva, who is sitting on the organizing committee for the Recovery of Biological Products XVII
…
Applications of Surface Plasmon Resonance for Detection of Bispec
October 22, 2015 - BioPharm Intl.
Surface plasmon resonance is helping define bispecific antibodies, the next-generation of biopharma therapeutics.
By Robert Karlsson
…
Changing the Culture of Cell Culture: Applying Best Practices and
October 22, 2015 - BioPharm Intl.
By Leonard P. Freedman, Mark C. Gibson, Richard M. Neve

Using Xcellerexâ„¢ mixing system as a slurry tank when packing ch
October 8, 2015 - Cytiva
 This application note describes the performance of single-use Xcell
This application note describes the performance of single-use Xcell
E. coli growth and Dab expression in single-use and stainless ste
October 8, 2015 - Cytiva
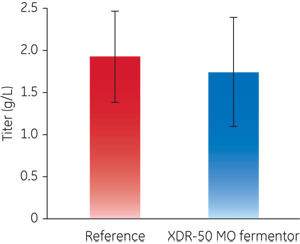 This application note describes the performance of the
This application note describes the performance of the