Avoiding Investigational Failures and Discrepancies
 Investigations-focused regulatory standards that apply to life-sciences organizations, such as FDA 21Code of Federal Regulations (CFR) 211.192, mandate that all deviations from written procedures must be thoroughly investigated and that the results of those investigations must be adequately documented. When life-sciences companies are unable to identify potential root causes and subsequently keep sufficient records of those events, their internal investigations are deemed incomplete by regulatory agencies.
Investigations-focused regulatory standards that apply to life-sciences organizations, such as FDA 21Code of Federal Regulations (CFR) 211.192, mandate that all deviations from written procedures must be thoroughly investigated and that the results of those investigations must be adequately documented. When life-sciences companies are unable to identify potential root causes and subsequently keep sufficient records of those events, their internal investigations are deemed incomplete by regulatory agencies.
The root of the problem
 The companies cited for these investigational failures are not alone. Historically, corrective actions and preventive actions (CAPAs) have been overused industry-wide as a part of the investigation process within the overall quality event management scheme. Even industry leaders have found themselves suffering from “death by CAPA” after cramming every quality event that occurs into their CAPA systems. It is becoming more evident that the key to effective and efficient investigations is to eliminate quality events as issues before escalating them from a correction to a corrective action. For this reason, the International Organization for Standardization (ISO) 9001 revised quality management standard has eliminated the term CAPA from its parlance and has now outlines separate processes for preventing action (PA) and nonconformance/corrective action (NCCA).
The companies cited for these investigational failures are not alone. Historically, corrective actions and preventive actions (CAPAs) have been overused industry-wide as a part of the investigation process within the overall quality event management scheme. Even industry leaders have found themselves suffering from “death by CAPA” after cramming every quality event that occurs into their CAPA systems. It is becoming more evident that the key to effective and efficient investigations is to eliminate quality events as issues before escalating them from a correction to a corrective action. For this reason, the International Organization for Standardization (ISO) 9001 revised quality management standard has eliminated the term CAPA from its parlance and has now outlines separate processes for preventing action (PA) and nonconformance/corrective action (NCCA).
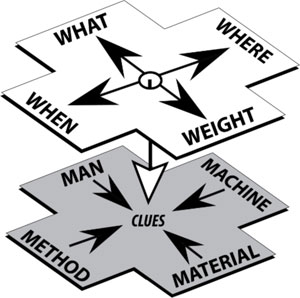
As referenced in FDA’s Quality Systems Approach to Pharmaceutical CGMP Regulations guidance, an “investigation” refers to the formal procedural process evaluation of a problem resolution (2). The Form 483 Investigational Observations issued most commonly for poor or ineffective resolutions are due to a poor or nonexistent investigation process by the offending -organization. All the data in the world won’t do any good if the process is inherently bad. By very definition, an investigation is the formal gathering of the factual boundaries of a problem or issue with the intention of uncovering clues which, by experiential treatment, are the source of generating the root cause (see Figure 1).
It should be noted that this investigational process demands the validation of action(s) to eliminate recurrence or proof that a plan of action will effectively address the resolution of an event. An action is not corrective if it cannot be proven to work on a repeatable basis and resolve the issue.
Finding out what you don’t know that you don’t know
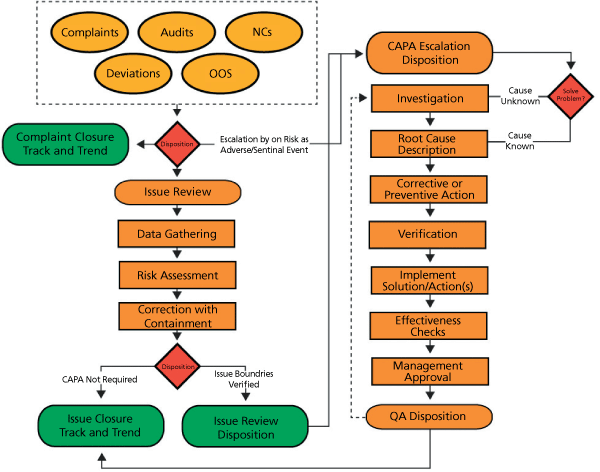
When done correctly and according to an established, proven process, an investigation can be as straightforward as following a road map. But, just like following a map, if you don’t know where you are, you won’t be able to figure out where you’re going. Figure 2 illustrates how a closed-loop quality event management system can act as a road map for investigations.
An investigation equation has three essential and interdependent components: clarification, decision making, and plan analysis.
Clarification. An investigation should start by thoroughly examining the input to clearly identify each respective issue of the event so that it may be completely addressed. These inputs come in the form of quality events such as customer complaints, deviations, nonconformances, out-of-specification incidents, and audits.
Decision making. The next potentially hazardous step where organizations can go wrong in their investigations is the decision-making phase where data are gathered and assessed. In this risk-based issue review phase an incident can be corrected or mitigated and closed, or a CAPA can be initiated, depending on what was discovered during the review. Each issue must be assessed for impact (negligible, minor, important, or critical) and frequency (rarely, frequently, or occasionally). Some issues may not be contained at the department level and must be escalated into a formal CAPA process. Different situations require different actions and, that being the case, the impact and frequency of the quality event can dictate what actions should be taken. A correction, for example, is ostensibly an immediate fix that can be made to eliminate an existing undesirable situation. For issues that can’t be resolved quickly, however, the company may carry out a series of corrective steps to determine the root cause(s) and prevent the reoccurrence of the problem(s).
Plan analysis. At the completion of the issue review phase, a documented plan analysis of the implementation is required, which gets back to the concept of knowing where one is in order to determine where one needs to be. The initiation of a CAPA marks the beginning of the third stage of a closed-loop quality event management system. The process of establishing corrective actions and preventive actions and effectiveness checks continues through this phase. It is during this investigational phase where root causes are conclusively determined, actions are implemented, and the event achieves closure once appropriate effectiveness checks have been finalized. Tracking and documenting these actions during this analysis phase is crucial to maintaining regulatory compliance.
Be aware, however, of an investigational truism that many working in regulatory environments have learned the hard way: for every solution, there is a cost. As such, several questions need to be taken into consideration when selecting the best action for eliminating a root cause:
- Are multiple components needed to achieve complete removal of the problem?
- Will the solution completely remove the root cause?
- Is there a possibility that the solution will cause other problems?
Once these questions have been answered, the best course for resolving the quality event comes into focus.
During the course of the investigation process, the guiding principle behind all efforts should be to find out exactly what you don’t know that you don’t know. Otherwise, the bulk of your efforts will just be guesswork.
EQMS: The key to avoiding investigational failures and discrepancies
An organization’s ability to integrate data and documentation from investigations with the rest of the company’s overall quality system is the foundational element of efficient quality management. Life-sciences companies can dramatically simplify their investigations and documentation of quality events by implementing an enterprise quality management system (EQMS) software solution that automates the handling of every phase of the quality event investigation process, from initiation, investigation, and all the way through to closure. An EQMS software solution that provides forms and workflow routes optimized according to industry best practices can provide a repeatable methodology for guiding quality teams through every step of quality event evaluations and CAPA implementation (i.e., identification of the problem, investigation of root cause, correction of problem, and prevention of recurrence). Such systems can be invaluable tools for implementing and sustaining dependable, repeatable, and compliant closed-loop investigation management protocols. Some robust customizable EQMS solutions even include functionality that automatically handles quality events in accordance with scalable preconfigurations and triggers CAPAs whenever established thresholds are met.
Other investigation-centered benefits of EQMS software solutions include:
- Automated routing, notification, delivery, escalation, and approval of CAPAs and all related documentation
- A central, secure repository for all documents pertaining to investigations
- Connections to all departments, product lifecycles, and quality processes
- Reduces duplicated efforts and data entry errors
- Simplified tracking, trending, and report creation
- Optimized quality processes and streamlined implementation of preventive measures.
Conclusion
Investigational failures and discrepancies can be avoided through the proper execution and documentation of investigations conducted by organizations that have the capability to securely maintain written records of their investigations within a central, audit-ready document management system. An EQMS can be integral to a life-science company’s ability to conduct and document investigations that meet even the stringent quality standards of global regulatory agencies.
References
1. FDA, Form FDA 483 Issued to Bausch & Lomb, Feb. 25, 2016. Accessed June 19, 2017.
2. FDA, Guidance for Industry, Quality Systems Approach to Pharmaceutical CGMP Regulations (CDER, September 2006).