 Design space is generally considered to be the areas where the product or process parameters can be run safely (without failure or high amounts of degradation) and achieve all critical quality attributes (CQAs), product and process acceptance criteria, and specifications. Knowledge of product or process acceptance criterion (specification limits) is crucial in design space generation and use. Design space is proposed by the applicant and is subject to regulatory assessment and approval. Movement within the characterized design space is not considered as a change and, therefore, allows for regulatory flexibility.
Design space is generally considered to be the areas where the product or process parameters can be run safely (without failure or high amounts of degradation) and achieve all critical quality attributes (CQAs), product and process acceptance criteria, and specifications. Knowledge of product or process acceptance criterion (specification limits) is crucial in design space generation and use. Design space is proposed by the applicant and is subject to regulatory assessment and approval. Movement within the characterized design space is not considered as a change and, therefore, allows for regulatory flexibility.
International Conference on Harmonization (ICH) Q8 (R2) 3.0 Glossary defines design space as follows (1):
“Design Space: The multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality. Working within the design space is not considered as a change. Movement out of the design space is considered to be a change and would normally initiate a regulatory post approval change process. Design space is proposed by the applicant and is subject to regulatory assessment and approval.”
Knowledge about the product and/or process is fundamental to product or process development. Knowledge about how X factors influence Y responses relative to CQAs is fundamental to defining and defending that knowledge. Ultimately, knowledge must be in the form of an equation (either empirical or based on well-established scientific principles) to be useful. Process and material equations are typically multiple factor, including main effects, interactions, and quadratic terms and may be either linear (in their coefficients) or nonlinear. Once the equation has been defined, it can be subsequently converted into a design space.
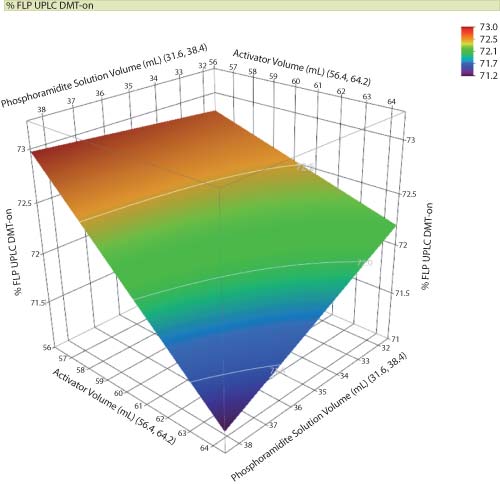
Design space is established through proper characterization techniques and is often an extrapolation of the response surface. Two-dimensional contour and three-dimensional surface plots are typically used in the visualization of the design space. Visualization, documentation, and communication of the design space helps to assure the product and or process set points are well defined and within safe and robust operating regions. Design space should include both material and process parameters relationships to acceptance criteria (CQAs) (see Figure 1).
Why Establish a Design Space?
There are three steps in the development of any product or process: system design, parameter design, and tolerance design. System design is the definition of the system (e.g., technology, API, excipients, cell line, methods, materials, equipment and/or sequence). Parameter design is the determination of all product and process set points and targets. Tolerance design is the allowable range that each X factor and Y response can be allowed to vary. Tolerance design can be done either with statistical distributions (when low risk and no safety issues) and/or with transfer functions (how X influences Y). Establishing a design space allows for the efficient determination and evaluation of all product and process set points and tolerance-design windows. Design space is the result of system design, the selection of all reagents, excipients, API concentrations, materials, equipment, process set points, and tolerances.