By Thomas A. Little, PhD
BioPharm International
Volume 28, Issue 7, pg 40–44
Approaches to the generation of process models, optimization techniques, and application of a design space are explored.
Developing product knowledge and process understanding is at the heart of modern drug development. Establishing a clear line of sight between critical quality attributes (CQAs), process parameters, and material attributes is a primary goal of drug development. Even though there are ICH guidance documents such as Q8 and Q11 that define what a design space is, there is still a poor understanding of the meaning and application of a design space.
ICH Q8, Pharmaceutical Development (1) defines a design space as: “The multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality. Working within the design space is not considered as a change. Movement out of the design space is considered to be a change and would normally initiate a regulatory post approval change process. Design space is proposed by the applicant and is subject to regulatory assessment and approval.“
This paper explores technically rigorous approaches to the generation of process models, optimization techniques for selection of set points, and application of a design space to defined CQAs and safe operational ranges.
Design of experiments (DOE) and other multivariate analysis techniques assist the developer in mapping out the design space and building process models. Once the DOE is complete, the developer can use the DOE to build a process model, define the design space, and run simulations for various optimums and to determine effective factor ranges where the out-of-specification (OOS) rates will be acceptable.
In reference to modern drug development ICH Q11, Development and Manufacture of Drug Substances states (2):
“Risk assessment can be used during development to identify those parts of the manufacturing process likely to have an impact on potential CQAs. Further risk assessments can be used to focus development work on areas for which better understanding of the link between process and quality is needed. Using an enhanced approach, the determination of appropriate material specifications and process parameter ranges could follow a sequence such as the one shown below:
• Identify potential sources of process variability.
• Identify the material attributes and process parameters likely to have the greatest impact on drug substance quality. This can be based on prior knowledge and risk assessment tools.
• Design and conduct studies (e.g., mechanistic and/or kinetic evaluations, multivariate design of experiments, simulations, modelling) to identify and confirm the links and relationships of material attributes and process parameters to drug substance CQAs.
• Analyze and assess the data to establish appropriate ranges, including establishment of a design space if desired.”
The following are generally accepted key steps for building a process model and using the model for development of product knowledge, process understanding, and regulatory submission:
1. State all CQAs of interest and their limits (upper specification limit [USL] and lower specification limit [LSL]).
2. Define the scale (small and/or at scale).
3. Define all processes and materials that will be used.
4. Complete a risk assessment (high level for all unit operations and low level for individual unit operations and materials).
5. Develop all single-factor and multiple-factor study designs and DOEs, include interactions and quadratics where indicated by the risk assessment.
6. Build the process model from the analysis of experimental data and determine all critical process parameters and critical material attributes.
7. Optimize the process and define the recipe and set points at their best value (robust optimization).
8. Evaluate the set points using the design space to evaluate margin.
9. Evaluate the design space using simulation and evaluate parts per million (PPM) OOS.
10. Set normal operating ranges and proven acceptable ranges with margin.
11. Verify the small-scale and at-scale results. Rescale the small-scale model to match the at scale process.
12. Define the effective design space used for process control and define the purpose of the design space.
Steps 7–12 will be discussed in detail in this paper.
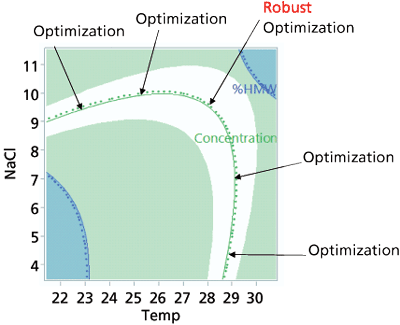
Figure 1: Robust optimization for a target concentration.
Step Seven: Optimize the process and define the set points
When determining the recipe for a formulation or process (set points) there are two methods that can be used. The first is optimization, and the second is robust optimization. Optimization works to find the best solution that meets all CQA requirements (see Figure 1), robust optimization works to assure the minimum transmitted variation occurs for all CQA goals. The difference in the two approaches is optimization works to achieve all goals and limits for all CQAs; robust optimization does the same but in addition it works to find the point in the design space where the first derivative (SAS/JMP) (see Figure 2) equals zero, also known as the sweet spot. Mathematically, the sweet spot is found where the first derivative of each response with respect to each noise factor are zero. Software programs such as SAS/JMP have these features built in. Robust optimization reduces variation at the operational target and is generally preferred over other optimization strategies.
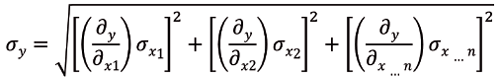
Figure 2: Partial derivative for robust optimization.
To achieve superior results and to find the robust optimum, two-factor interactions and quadratic terms must be included in the model. Main effects, quadratics, and interactions must be considered during the risk assessment and part of the DOE design. Main effects only and/or screening type experiments will not result in a robust solution.
Step Eight: Evaluate the set points using the design spaceOnce the set points have been selected, the visualization of the design space can be generated. Every DOE can create a design space. Care needs to be exercised in understanding and interpreting a design space. The visualization of the design space is of the mean (average) in the response surface (see Figure 3) relative to the limits of the CQAs. Many think that being anywhere in the white space will achieve a good result and being in the colored or shaded area is bad. That is an incorrect interpretation of the graph. Just being in the white area is no assurance that each batch, vial, or syringe will be in specification, only that the average from the process model will be within the limits. Also any visualization of the design space is static; the actual design space is dynamic depending on the settings of the other factors. Only simulation (3) can explore and evaluate settings within the design space, examine potential failure rates, and evaluate the dynamic nature of the process.
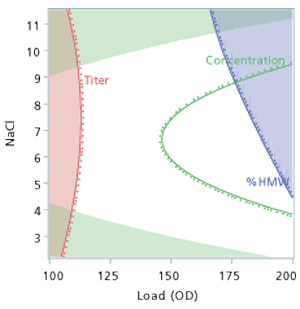
Step Nine: Evaluate the design space using simulation
To simulate batch-to-batch, unit-to-unit, or vial-to-vial variation at the set point, Monte Carlo simulation is used. OOS capability in PPM should be targeted to less than 100 for each CQA or lower. The simulation includes three key sources of variation: the mathematical expression or model from the characterized product or process; variation of each factor at the targeted set point; and the residual variation not accounted for by the model (4). The residual variation is the root mean squared error (RMSE) from the model and includes the variation from the analytical method as well as any other uncontrolled factor when building the model.
A good understanding of the process or equipment capability will aid the developer in building the simulation (see Figure 4). Normal, truncated, and non-normal distributions are used to inject the simulated noise plus the RMSE and reflect it onto the model to predict the CQA response. The more accurately the variation at set point is understood, the more accurately it will reflect OOS release rates of drug substance or drug product.
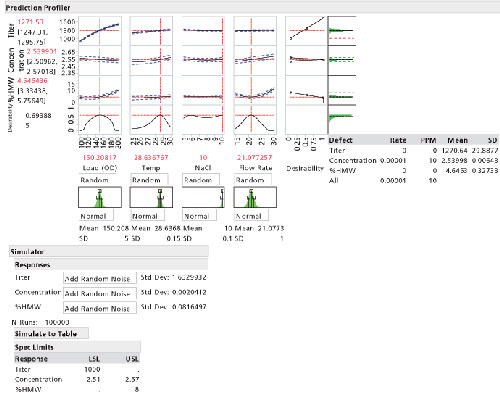
Step Ten: Set normal operating ranges and proven acceptable ranges
To evaluate normal operating ranges (NOR) and proven acceptable ranges (PAR), the simulation injects variation at set point, 3 sigma, 4.5 sigma, and 6 sigma ranges (see Table I) are typically evaluated for their associated PPM. Normal, non-normal, actual re-sampling from measurements, and uniform distributions can be used to evaluate PPM rates. Typically, the limits are set to assure the CQA PPM failure rates are below 100. Uniform distributions should be used if processing to range; normal distributions are typically used when processing to target; however, other distributions are possible based on the product and the problem.
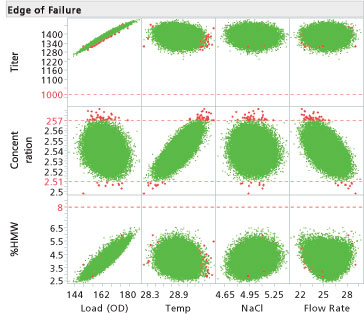
Step Eleven: Verify the small-scale and at-scale results
Verification and validation (5) runs at the robust optimum are performed to verify the model prediction and the actual measurements are in agreement. Typical acceptance criteria confirm the small- or at-scale measurements are within the 99% quantile interval from the simulated results. If there is a detected shift between the small-scale and at-scale data, the model can be rescaled/calibrated to match the at-scale results. Some mechanistic understanding of the scale difference is generally recommended when scale effects are detected.
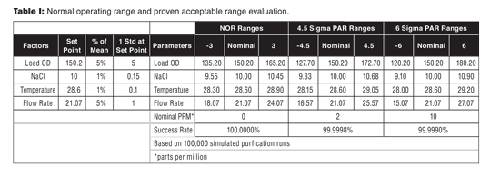
Table I: Normal operating range and proven acceptable range evaluation.
Step Twelve: Define the effective design space that will be used for process control
Finally, there is a difference between the visualization of the design space and the effective design space an applicant may want to file with the health authorities. The effective design space is the region where no OOS events occur and/or the applicant will adjust to correct for processing conditions, raw material potency, and/or dose or formulation requirements. In most cases, the effective design space is much smaller in range than the visualized design space.
Summary
Knowing how to complete a risk assessment and design an appropriate experiment are only two key steps in a series of development activities. Knowing how to complete the development, select the robust optimum, simulate potential OOS rates for all CQAs, determine and evaluate design margin, find the NOR and PAR limits, and define and defend the effective design space are essential skills that all those that work in drug development. These skills should be gained by instruction and by practical experience working on drug substance and drug product and with the health authorities on filing the design space and associated control plans.
ALL FIGURES ARE COURTESY OF THE AUTHOR.
References
1. ICH, Q8, Pharmaceutical Development (ICH, November 2005).
2. ICH, Q11, Development and Manufacture of Drug Substances (ICH, November 2012)
3. SAS/JMP Software, Profilers and Simulation, Version 12, May 2015
4. T. Little, Robust Optimization, Design Space and Tolerance Design (Course Notes, 2015)
5. FDA, Process Validation, General Principles and Practices (FDA, January 2011).
Article Details
BioPharm International
Vol. 28, No. 7
Pages: 40–44
Citation:
When referring to this article, please cite it as T.A. Little, "Robust Optimization, Simulation, and Effective Design Space," BioPharm International, 28 (7) 2015.