Monoclonal antibodies (mAb) increasingly form the majority share of the product pipeline, as well as revenue, of major biopharmaceutical companies (1). What is unique about mAbs from a processing perspective is the applicability of developing a common process and analytical platform (2, 3). Some process development related attributes with respect to production of mAbs include:
• Significant increase in titer levels over the past decade (from 0.5 mg/mL to 5-10 mg/mL)
• High product requirement (as much as tons) due to higher doses of typical mAb products
• Larger manufacturing facilities (from 1-5KL to 10-20KL)
The efficiency of the platform process, as it enables process development (e.g., time taken, resources required), analytical development, and manufacturing, is quite significant, such as:
• Process development timelines can be significantly reduced
• Analytical development timelines can also be significantly reduced (use of standardized assays and platforms)
• Ease of scale-up and technology transfer due to similarity in the processes
• Reduced capital expense when bringing in a new product to the manufacturing facility.
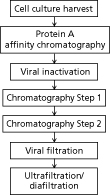 Traditional Monoclonal Antibody Platform
Traditional Monoclonal Antibody Platform
Figure 1 illustrates the traditional mAb platform that is commonly used (2,3). Typical steps include:
• Protein A chromatography for capture of the product and removal of host cell-related impurities (host cell proteins and DNA). Though extremely efficient and effective, the step primarily suffers from the significantly high cost of Protein A resins in comparison to other popular modes of chromatography (such as ion exchange). The low pH elution is another potential issue as it has been linked to product aggregation.
• Low pH viral inactivation as an orthogonal step for clearance of retroviruses. This step also suffers from the possibility of product aggregation at low pH.
• Cation exchange (CEX) chromatography is also quite commonly used. The primary objective of this step is to remove host-cell proteins, DNA, charged variants, and aggregates. Bind and elute mode is used to facilitate removal of product-related impurities.
• A second chromatography step is often used (either prior to the CEX step or following it) with the purpose of further removal of host-cell related impurities (e.g., host-cell proteins and DNA) or product-related impurities. Anion exchange (AEX) chromatography in a flow-through mode is the method of choice for removal of host-cell impurities. Hydrophobic-interaction chromatography (HIC) in bind and elute mode has been used to achieve further clearance of product-related impurities.
• Virus filtration step is the method of choice as another orthogonal step for virus removal.