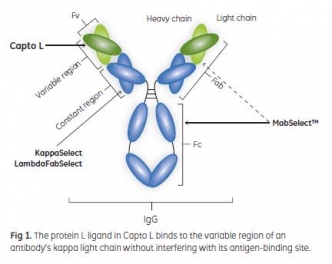
 This Application note describes a three-step purification process of a domain antibody (Dab) expressed in the periplasm of E. coli. First, a capture step using Capto™ L was used to reduce E. coli host cell proteins (ECP) and endotoxin levels.
This Application note describes a three-step purification process of a domain antibody (Dab) expressed in the periplasm of E. coli. First, a capture step using Capto™ L was used to reduce E. coli host cell proteins (ECP) and endotoxin levels.
Next, a polishing step using Capto MMC ImpRes further reduced ECP and endotoxin levels at yields of > 86%.
Finally, Capto adhere ImpRes was run in flowthrough mode to render product with undetectable endotoxin levels
and ECP content of < 10 ppm. All purification steps were optimized by Design of Experiments (DoE), thereby supporting a Quality by Design (QbD) approach.
Finally, Capto adhere ImpRes was run in flowthrough mode to render product with undetectable endotoxin levels
and ECP content of < 10 ppm. All purification steps were optimized by Design of Experiments (DoE), thereby supporting a Quality by Design (QbD) approach.
Using Capto L, Capto MMC ImpRes, and Capto adhere ImpRes media in the three step process resulted in efficient removal of the main contaminants and strong yields (> 80%) over the entire process. This platform approach, based on a capture step with Capto L, enables increased efficiency and productivity in developing therapeutics based on Dabs.