Although the phrase QbD has been thought of as a new or borrowed concept and discussed in terms of feasability, many CMOs have been using these concepts, albeit perhaps not in a well-planned, cohesive manner. Now the discussion has turned to breaking down the reality of a QbD strategy. Implementing the infrastructure of a QbD approach to process development involves formalizing the commitment and the strategy and integrating execution into quality systems. The end result is a road map for the development of the product from the very beginning.
The purpose of this paper is to present a scenario based upon a real case study, where the project decision making followed a "traditional" approach to development and commercialization, which in turn caused longer development timelines and increased program costs. In this example, the traditional approach involved minimizing development studies to keep costs down, waiting for clinical data to perform any further optimization, and then performing a long series of univariate characterization studies to complete the information required for a submission. In contrast to this approach, a second product with a different client was planned and scheduled against a timeline with a built-in strategy to gain knowledge of the process more quickly. The predicted result not only lowers the risk of the manufacturing commercialization effort, but should also be a significant time savings. For a CMO, occupying space in manufacturing or even on the bench costs money; thus, time is money in the truest form.
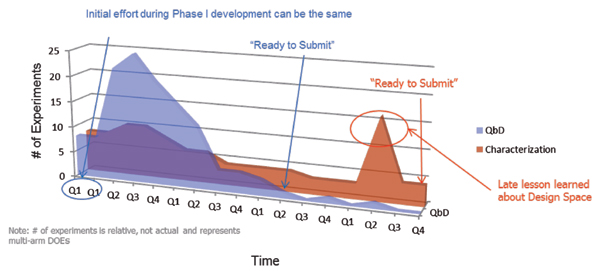
Figure 1 illustrates the difference in approaches. Both products are similar in complexity; therefore, the total knowledge required to be "ready to submit" (i.e., the area under the curve in Figure 1) is relatively constant between the two products. The timeline presented in Figure 1 represents time spent in product development at the CMO, not necessarily real time. What it does demonstrate, however, is that using the aforementioned QbD model, the CMO portion of the product commercialization is unlikely to ever be the bottleneck for a commercialization timeline. Additionally, the smarter portion of the QbD approach is minimizing redundant or inefficient characterization studies and reducing the risk of having to repeat characterization based upon late understanding of the process.