Biosimilars to Drive Modern Manufacturing Approaches
December 9, 2016 - Pharm Tech
Reliable, high-quality products require innovative analytics and production.
By Jill Wechsler
Managing Risk in Raw Material Sourcing
December 9, 2016 - Pharm Tech
Vendor selection and materials testing are complex enough, but in today’s volatile environment, risk mapping and monitoring are also crucial.
…
Your Protein Purification Questions Discussed with Cytiva R&D Sci
December 8, 2016 - Cytiva
 Within ÄKTA club, web chats are held. In the web chats, ÄKTA sys
Within ÄKTA club, web chats are held. In the web chats, ÄKTA sys
Protein Characterization Using SEC: Three Webinar Takeaways
December 8, 2016 - Cytiva
 The possibility to separate molecules by size under n
The possibility to separate molecules by size under n
Automating Processes in Upstream Processing
December 8, 2016 - BioPharm Intl.
By Susan Haigney
BioPharm International spoke with Trevor Marshall, director of enterprise systems integration at
…
Efficient Purification of Pneumococcal Polysaccharides in a Chrom
November 28, 2016 - Cytiva
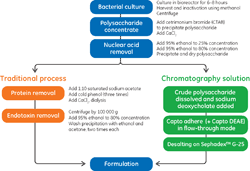 This application note demonstrates the purificatio
This application note demonstrates the purificatio
Efficient Purification of the Pertussis Antigens Toxin, Filamento
November 28, 2016 - Cytiva
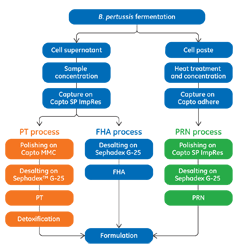 This application note describes the purification of pertussis tox
This application note describes the purification of pertussis tox
Advancing Single-Use Technology Through Collaboration
November 11, 2016 - BioPharm Intl.
By working together to harmonize the highly variable steps within the biopharmaceutical manufacturing process, both end users and suppliers are making s
…
Validation of the production of influenza virus in ReadyToProcess
November 10, 2016 - Cytiva
 This application note describes the validation of the single-u
This application note describes the validation of the single-u
A Platform Approach to Purification of Antibody Fragments
November 10, 2016 - Cytiva
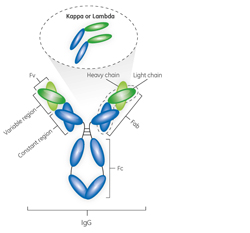 Antibody fragments constitute a promising class of biopharmac
Antibody fragments constitute a promising class of biopharmac
