Development of column packing methods based on pressure flow meas
November 10, 2016 - Cytiva
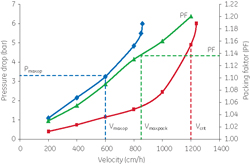 Scale-up of a chromatography process might appear
Scale-up of a chromatography process might appear
Walk Away and Do More Screening Preps
November 10, 2016 - Cytiva
Developing a process or looking for a clone of interest often requires screening large numbers of samples. Many analyses require purified protein, and substanti
…
Improving Process-Scale Chromatography
November 10, 2016 - BioPharm Intl.
Advances in technology are increasing the productivity and efficiency of commercial-scale chromatography bioprocesses.
By Cynthia Ch
…
Defining Risk Assessment of Aseptic Processes
October 26, 2016 - PharmTech
Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the assessment of risk in the processing of intravenous injectable d
…
Real Time Continuous Microbiological Monitoring
October 10, 2016 - BioPharm Intl.
Laser-induced fluorescence, a rapid microbiology method for real-time airborne particle and microbial monitoring, enhances sterility assurance in pharma
…
Establishing Acceptance Criteria for Analytical Methods
October 10, 2016 - BioPharm Intl.
Knowing how method performance impacts out-of-specification rates may improve quality risk management and product knowledge.
By Thom
…
Mixing dynamics in a large volume single use mixer
September 29, 2016 - Cytiva
 Large volume single-use technology mixers can be used f
Large volume single-use technology mixers can be used f
High-Throughput Process Development Handbook
September 29, 2016 - Cytiva
Time-to-clinic and time-to-market are two key factors for successful biopharmaceutical development. Efficient development of the manufacturing process i
Modeling Bioreactor Performance
September 29, 2016 - BioPharm Intl.
Model effectiveness is determined by the quality and composition of the data inputs.
By Cynthia A. Challener
Efforts Accelerate to Streamline Postapproval Change Process
September 29, 2016 - Pharm Tech
Manufacturers and regulatory authorities seek coordinated lifecycle management policies.
By Jill Wechsler