Developing oligonucleotide therapeutics with confidence
November 17, 2023 - Cytiva
In this video, we will look at
Good modeling practice: workflow and case study
November 17, 2023 - Cytiva

Learn more about a recommended mechanisti
…
Top challenges in recombinant protein purification process develo
November 17, 2023 - Cytiva, BioPharm International
…
Discussing the Origins of the mRNA Therapeutics Field
November 17, 2023 - BioPharm International

Andy Geall, co-founder and chief development officer at Replicate
…
Chromatography processes for the diversified therapeutic antibody
November 3, 2023 - Cytiva
The diversity of antibody variants in today’s pipeline presents unique challenges for purification and we see molecules such as bsAbs, Fabs, dAbs, and others.
…
Process Development for Viral Vectors
November 3, 2023 - Cytiva

Scaling viral vector processes can be challenging, but it doe
…
Digitalization: The Route to Biopharma 4.0
November 3, 2023 - BioPharm International
Efficient and Scalable Buffer Preparation
November 3, 2023 - Cytiva
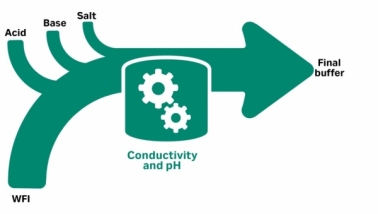
This article desc
Ready for a strong start in oligo manufacturing?
October 20, 2023 - Cytiva

Are you interested in manufacturing your own oligonucleotide therap
…
Virus safety challenges: get the job done!
October 20, 2023 - Cytiva

Virus safety is key in bioprocessing. In this free 1-day semina