Alternate capture step
Although Protein A chromatography is a dominant capture step employed in the mAb platform process, it has several major drawbacks. Apart from the relative high cost of the resin, it has limited binding capacity and issues with clean-in-place (CIP) with sodium hydroxide. The alternative can be cation exchangers or hydrophobic charge induction chromatography (HCIC) as a capture step.
Various authors have described cation-exchange chromatography as a capture step in mAb purification (8-11). Though it may not be as efficient as Protein A in terms of selectivity of HCP, it can be used in combination with effective HCP precipitation step. Additionally, to gain similar HCP clearance compared to Protein A, it can be used along with anion exchangers followed by HIC. With the evolution of mixed-mode resins such as Capto Adhere (Cytiva), Ceramic hydroxyapatite (Bio-Rad Laboratories), HEA HyperCel (Pall Life Sciences), and MEP HyperCel (Pall Life Sciences), selectivity can be further enhanced to match Protein A resin (12). One direct advantage of not using Protein A resin is getting rid of leached Protein A and increased life cycle of the capture step. Recent developments have led to availability of cation exchange media with dynamic binding capacities of >100 grams of protein per liter of resin (8). Several commercial processes have been successfully developed using this approach (2).
One major disadvantage of cation exchange as a capture step is the need to precondition the harvest at lower ionic content to promote binding. Though some of the new generation cation exchangers offer high salt binding at pH range of 5 to 6 (11) an alternate is use of HCIC as a capture step (8). This helps in eliminating preconditioning of load from cell-culture harvest. The product can be eluted at lower pH similar to Protein A. Currently, it offers relatively lower binding capacity than Protein A resin but can be a viable option considering the lower cost, high reuse cycle, and lower process development cost in comparison to cation exchange as capture step.
Use of non-chromatographic unit operations
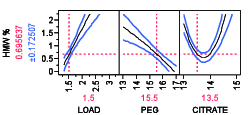
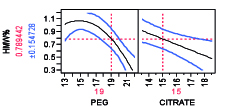
Non-chromatographic unit operations, such as the aqueous two-phase separation, have been long proposed and investigated as an alternative to process chromatography for the purification of mAb products due to its cost effectiveness, high capacity, biocompatibility, and scale up potential (13). Aqueous two-phase extractions (ATPS) that have been proposed for production of mAb products including PEG-phosphate, PEG-citrate, PEG-dextran, and PEG-dextran (14,15). ATPS has been shown to be effective for reduction of low molecular weight (LMW) and high molecular weight (HMW) mAb aggregates (16). Removal of HMW as a function of PEG-citrate concentration, for example, is shown in Figure 6 for two different mAbs. The profiler shown for the model is constructed using design of experiment with varying PEG and citrate weight percentage. It is possible to remove HMW completely by appropriate mixture percentage of PEG citrate at pH 7.0 though the trend may vary from mAb to mAb. A recent study has compared ATPS to the currently established platforms in terms of costs and environmental impact (16). The authors reported that the ATPS offers considerable advantages in terms of process economics, especially when processing high titer cell-culture supernatants. For a given amount of mAb product manufactured, the ATPS-based process reduced the annual operating cost by 40% when cell-culture supernatants with mAb titers higher than 2.5 g/L are processed.
Membrane Chromatography
Membrane chromatography is another emerging alternative to the traditional packed-bed chromatography (17,18). The key benefits it offers are the elimination of diffusive pores and making the mass transfer of the protein to binding sites convective rather than diffusive. The use of convective transfer results in relatively high operational flow rates. Membranes with a variety of ligands (e.g., affinity, ion exchange, hydrophobic interaction) are available on the market. The most popular application involves use of anion-exchange membrane as a polishing step in the mAb purification platform. Such membranes have been shown to be effective in flow-through mode to remove trace amounts of impurities. In a recently published study, the authors demonstrated the feasibility of using hydrophobic interaction membrane chromatography for removal of aggregates and leached Protein-A for a mAb product (19). Due to higher hydrophobicity, the aggregates were removed by selective adsorption on the membrane. Protein-A was removed as it formed relatively hydrophobic complexes with the mAb. Thus, the mAb product eluted in the flow-through while the impurities remained bound to the membrane and could subsequently be eluted by lowering the salt concentration. With the high throughput that membrane adsorbers provide, this could be a promising addition to the antibody purification platforms.
Summary
It is evident that many alterations to the mAb platform process have been implemented by biopharmaceutical companies or are under consideration. With the advent of biosimilars and the related increased pressure on lowering the cost of production, it is likely that the next decade will see more significant changes in the platform.
References
1. The Development of Therapeutic Monoclonal Antibody Products, Editors: H. L. Levine and G. Jagschies (BioProcess Technology Consultants Inc. and General Electric Company, 2010).
2. A.A. Shukla, B. Hubbard, T. Tressel, S. Guhan, and D. Low, J. Chromatogr. B 848, 28-39 (2007).
3. J. Glynn, T. Hagerty, T. Pabst, G. Annathur, K. Thomas, P. Johnson, N Ramasubramanyan, P Mensah. BioPharm Intern. Supplement (2009).
4. G. Rao et al., 243rd ACS National Meeting, BIOT 83, 2012.
5. B. Kelley et al., Biotech and Bioengg., 101 (3), 2008.
6. H. F. Liu, J. Ma, C. Winter and R. Bayer, mAbs, 2, 480-499 (2010).
7. P. Gagnon, Purification of Monoclonal Antibodies by Mixed-Mode Chromatography, in Process Scale Purification of Antibodies, Ed. U. Gottschalk (John Wiley & Sons, Inc., Hoboken, NJ, 2008).
8. G. M. Ferreira, J. Dembecki, K. Patel, and A. Arunakumari, Biopharm Intern., 20(5) (2007).
9. M. Urmann et al., Bioscience In MAbs, 2 (4) pp. 395-404 (July 2010).
10. S. Andreas, and A. Kiesewetter, J.Chromatogr. B 848.1 151-158 (2007).
11. Lain, Blanca, A. M. Cacciuttolo, and G. Zarbis-Papastoitsis, BioProcess International (2009).
12. J.Chen et al., J.Chromatogr. A, 1217(2), 216-224 (2010).
13. I.F. Ferreira et al., J.Chromatogr. A, 1195(1-2), 94-100 (2008).
14. L. N. Mao et al., Biotechnology Progress, 26(6), 1662-1670
15. P. A. J. Rosa, I. F. Ferreira, A. M. Azevedo, and M. R. Aires-Barros, J.Chromatogr. A, 1217 (16), 2296-2305 (2010).
16. N. Fraud et al., BioProcess Intern., June, 30-35 (2009).
17. P.A.J. Rosa, A.M. Azevedo, S. Sommerfeld, W. Bäcker, M.R. Aires-Barros, Biotechnology Advances, 29, 559-567 (2011).
18. A. S. Rathore and A. Shirke, Preparative Biochemistry and Biotechnology, 41, 398-421 (2011).
19. S. M. Yoo and R. Ghosh, Journal of Membrane Science, 390-391, 263-269 (2012).