Operational Excellence: More Than Just Process Improvement
October 13, 2013 - Ian Uydess, Robert E. Spector, BioPharm International Magazine
By embracing efficiency and quality, biopharmaceutical organizations can work better and achieve better work.
Life-sciences companies
…
Essentials in Establishing and Using Design Space
October 4, 2013 - Thomas A. Little, PhD BioPharm International
Knowledge of product or process acceptance criterion is crucial in design space.
Report from the Eighth International Plasma Product Biotechnology
October 3, 2013 - Cytiva

Biosimilars Development and Supply: How Complex Can the Process B
September 30, 2013 - Martin Van Trieste, BioPharm International Magazine
As the complex requirements of manufacturing biologics are manifold, it is important that biomanufacturing companies adopt quality-by-design principles.
…
Elucidating Biosimilars Characterization
September 30, 2013 - Adeline Siew, PhD. BioPharm International Magazine

…
A Platform Approach for the Purification of Domain Antibodies (Da
September 30, 2013 - Cytiva
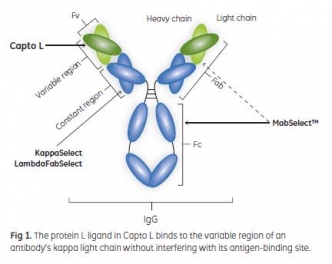 …
…
The use of Xcellerexâ„¢ mixing system as slurry tank when packing
September 23, 2013 - Cytiva
…
Prescribing Caution for Biosimilars
September 20, 2013 - James C. Greenwood, Biopharm International Magazine
 In statehouses around the country, lawmakers are beginning to
In statehouses around the country, lawmakers are beginning to
HT Multi-Product Liquid Chromatography for Characterization of Mo
September 16, 2013 - Jennifer C. Rea, PhD, G. Tony Moreno, Yun Lou, Rahul Parikh, Dell Farnan, PhD , Biopharm Internation
If used correctly, these new analytical methods can reduce analysis and product development time.
…
The Development and Application of a Monoclonal Antibody Purifica
September 16, 2013 - By: Judy Glynn, Timothy Hagerty, Timothy Pabst, PhD, Gopinath Annathur, Kristin Thomas, Paul Johnson
A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase the
…
