Single-use Bioreactors Have Reached the Big Time
April 5, 2017 - BioPharm Intl.
The decision to use disposable bioreactors is now driven by commercial rather than technological considerations.
By Cynthia A. Chall
…
Cost Considerations Drive Lean Technology in Biopharmaceutical Ma
April 5, 2017 - BioPharm Intl.
By Catherine Shaffer
Efficient concentration of a low-titer bovine IgG with high recov
March 23, 2017 - Cytiva
Reconciling Sensor Communication Gaps
March 22, 2017 - BioPharm Intl.
By Angelo DePalma, Ph.D.
High Throughput Protein Purification at a Glance
March 22, 2017 - Cytiva
 Within the Human Protein Atlas project, scientists are using
Within the Human Protein Atlas project, scientists are using
Automating Bioprocesses
March 9, 2017 - BioPharm Intl.
Automation of stainless-steel systems and single-use systems differs in complexity.
By Jennifer Markarian
Ensuring Sterility in Small-Scale Production
March 8, 2017 - PharmTech
Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses how to ensure sterility when manufacturing small-scale parenteral ba
…
Simplify Every Step of Histidine-Tagged Protein Purification
March 8, 2017 - Cytiva
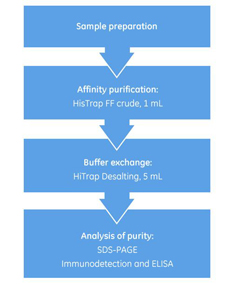 A purity between 60% an
A purity between 60% an
Ensuring the Biological Integrity of Raw Materials
February 23, 2017 - BioPharm Intl.
By Catherine Shaffer

Impact of Manufacturing-Scale Freeze-Thaw Conditions on a mAb Sol
February 23, 2017 - BioPharm Intl.
The objective of this study was to assess the impact of manufacturing-scale, freeze-thaw conditions on aggregation and subvisible particle formation of
… This
This