Overcoming Buffer Challenges With In-Line Conditioning
September 14, 2016 - Cytiva
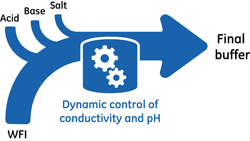 Preparation and storage of the large number of buffers requi
Preparation and storage of the large number of buffers requi
Process Chromatography Selection for Downstream Processing Applic
September 14, 2016 - BioPharm International
Industry experts discuss best practices for selecting a separation technology.
By Caroline Hroncich
Safety Drives Innovation in Animal-Component-Free Cell-Culture Me
September 14, 2016 - BioPharm Intl.
Advances in cell culture media technology have helped achieve safer biologics.
By Tom Fletcher, Holden Harris
Innovation vs. Capacity: How CMOs Compete
September 14, 2016 - BioPharm Intl.
The strategies of an innovation-driven CMO may be different than a capacity-driven CMO.
By Jim Miller
Perfusion in the 21st Century
August 31, 2016 - BioPharm Intl.
Quality, flexibility, and cost savings are driving use of perfusion technology in biosimilars manufacturing.
By Bruce Lehr, Delia Ly
…
Technology solutions simplifying chromatography unit operations
August 31, 2016 - Cytiva
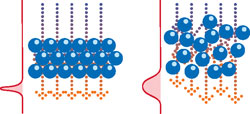 Chromatography operations typically involve steps
Chromatography operations typically involve steps
Simplify chromatography method creation
August 31, 2016 - Cytiva
 Method creation is an essential part of preparing for automated pr
Method creation is an essential part of preparing for automated pr
A Q&A with Eric Langer: CMOs Embrace New Technology
August 29, 2016 - Process Development Forum
Process Development Forum speaks with Eric Langer, President and Managing Partner at BioPlan Associates, and the author of a number of biopharma studies, ab
…
Analysis of Glycosylation in Biosimilars
August 17, 2016 - BioPharm Intl.
A step-wise process is used to characterize glycans and understand the functioning of a molecule for biosimilar development.
The str
…
Generating a Fully Processed Antibody
August 17, 2016 - BioPharm Intl.
The authors outline cell-line development and process scale-up for an antibody program in which the antibody requires additional processing by a site-sp
…
